Prostate Cancer Detection: Complexities and Strategies
Julie-Ann O’Reilly1*, Richard J. O’Kennedy1,2
Abstract
Prostate cancer (PCa) incidence is increasing due to the ageing worldwide population. In recent years, the unsuitability of prostate specific antigen (PSA) as a diagnostic biomarker has become apparent. Unnecessary treatment of men with non-lethal disease leading to significant quality-of-life reducing side-effects resulted in 2017 in the US Preventative Services Task Force (USPSTF) recommending selective and limited use of PSA testing for PCa. An immediate need exists for a test capable of accurate and early diagnosis of PCa. Critically, this test must differentiate between indolent and aggressive PCa to inform appropriate management of the disease. The key to predictive accuracy is the utilisation of combinations of diverse biomarkers and clinical data. A number of suitable new tests have emerged since 2013 but none of these have been implemented in the clinical setting. Extensive clinical validation of these tests is critical to address the chasm that now exists in PCa diagnostics. This mini review assesses current clinical issues for PCa diagnosis, evaluates currently available commercial tests and critically assesses next-generation diagnostic tools that could revolutionise PCa diagnostics.
Introduction
Prostate cancer (PCa) was first described in the 19th century by J. Adams and reported as ‘a very rare disease’1. Now, in the 21st century, it has become the major cancer diagnosed in men. The morbidities associated with mistreatment of PCa are a critical concern due to the significant resulting reduction in quality-of-life2. Additionally, although the most common cancer diagnosed in men, it is the second most common cause of cancer-related mortality in men indicating the importance of both diagnostic and treatment options3. PCa is a global health concern; however, incidence and mortality are not directly correlated and the differences in survival rates due to geographical location and economics are significant highlighting the importance of early diagnosis for positive prognosis4,5 (Figure 1). PCa cancer diagnoses are projected to increase to 2.1 million by 2035 with up to 633,328 associated deaths predicted as a result of the worldwide increase in life-expectancy6. Survival is directly related to stage-at-diagnosis and therefore investing in disruptive and effective diagnostic tools would lead to a reduction in mortality. Awareness of the detrimental effects of treating PCa patients unnecessarily, and the compromised utility of the use of prostate-specific antigen (PSA) detection as an informative diagnostic tool, have led to increased focus on the identification of novel biomarkers and the development of better diagnostics. Translation of these novel biomarkers and assays into informative clinical diagnostics to aid in PCa screening programmes fundamentally still requires significant further input and clinical validation.
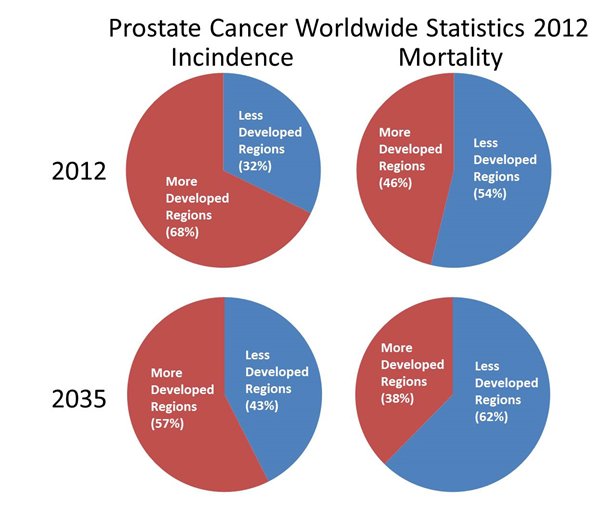
Figure 1: Estimates of Incidence and Mortality of Prostate Cancer for 2012 and 2035 in more developed regions (More Dev Reg.) and less developed regions (Less Dev Reg.).
| Actual Estimated Numbers | ||||
|---|---|---|---|---|
| Less Developed Regions | Less Developed Regions | |||
| 2012 | 2035 | 2012 | 2035 | |
| Incidence | 352950 | 767412 | 741966 | 1036965 |
| Mortality | 165467 | 382297 | 142014 | 230816 |
Data Source GLOBOCAN 2012. http://globocan.iarc.fr/Pages/burden_sel.aspx [Internet]. [Accessed 26/10/2017]. More developed regions include all regions of Europe plus Northern America, Australia/New Zealand and Japan. Less developed regions include all regions of Africa, Asia (excluding Japan), Latin America and the Caribbean, Melanesia, Micronesia and Polynesia.
This mini review will provide an overview of the history of PCa diagnosis and the lessons learned from reliance on a single diagnostic biomarker, PSA. It will describe the current diagnostic pathway, evaluate currently available commercial tests and critically assess next-generation diagnostic tools.
History of Prostate Cancer Diagnosis
PCa is a complex, heterogeneous disease that is generally asymptomatic in the early stages and consequently is a highly challenging diagnostic issue for clinicians and men worldwide. The majority of men may experience great discomfort and embarrassment with any investigations of the prostate other than a blood test or provision of a urine sample. Prior to the 1970s, the majority of PCa cases were diagnosed by digital rectal examination (DRE) and were symptomatic and advanced7. All techniques currently except serum PSA or provision of a urine sample. DRE is the oldest and most commonly used screening test for PCa and investigates the prostate gland for the presence of lumps or hardening. Although it remains a key step in the pathway to PCa diagnosis, studies have found that it has little or no effect in preventing metastatic PCa or reducing mortality to any appreciable degree8,9. This is a fundamental issue for initial PCa diagnosis and highlights the need for better early diagnostic procedures. Greyscale trans-rectal ultrasound (TRUS), originating in the 1970s, was further developed to TRUS-guided biopsy in the 1980s. TRUS-guided biopsy is used for the systematic acquisition of 10-12 cores per prostate gland and is more sensitive and specific than greyscale TRUS7. It remains one of the main techniques used today in assessing cancer of the prostate. Prostate biopsy is the only conclusive method of confirming the presence of PCa10.
PSA - from champion to outcast and back again
PSA, a serine protease synthesized by the prostate tissue, responsible for seminal fluid liquefaction, was hailed as the gold standard biomarker for PCa detection for nearly two decades11 (Figure 2). However, pathologist Richard Albin who discovered PSA back in 1970 has written a book entitled ‘The Great Prostate Hoax: How big medicine hijacked the PSA test and caused a public health disaster’ where he lists major concerns about using PSA as a screening tool for PCa12. Primarily, his concerns are, that PSA is not cancer-specific and therefore not diagnostic; and that PSA levels give no information about whether a cancer is indolent or aggressive. Albin believes that PSA is only useful as an indicator of PCa recurrence13. Nevertheless, serum PSA was approved in 1994 for PCa screening in conjunction with DRE by the US Food and Drug Administration (FDA)14. Attempts to increase the specificity and sensitivity of PSA assays by using different PSA isoforms and altered PSA glycosylation patterns have considerably improved the accuracy of PSA testing15. In spite of this, in 2012, as a consequence of over-diagnosis and the inability of PSA testing to discriminate between indolent and aggressive forms of the disease, the US Preventative Services Task Force (USPSTF) published a recommendation against the use of PSA for diagnosis of PCa16. Consequently a 3-10% decline in screening rates across men of all ages was observed. Additionally, reported PCa incidence and biopsy declined concurrently. However, this decline in screening has led to subsequent detection of more advanced disease with a higher proportion of tumours of higher grade and stage being detected17. In 2017, the USPSTF published a recommendation supporting the selective use of PSA testing following increased evidence that it can reduce PCa-specific mortality18. Currently, the debate continues as to the utility of PSA. Although its determination may reduce prostate cancer mortality, the key question is ‘at what cost?’ to the survivor.
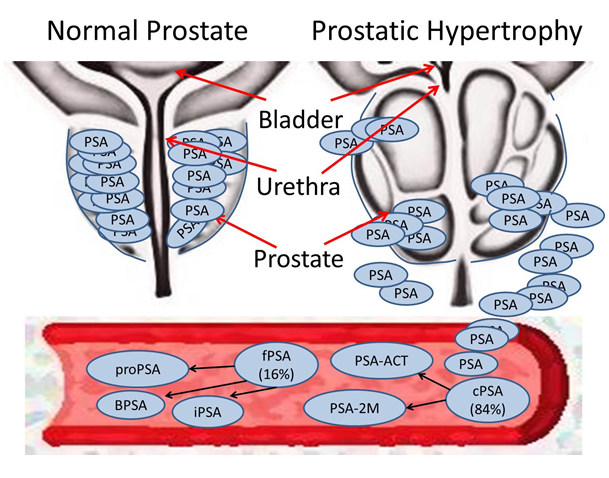
Figure 2: Prostate Specific Antigen (PSA) is released from the prostate gland following perturbations of the cellular microenvironment.
Complex PSA (cPSA) accounts for 84% of total PSA (tPSA) and can be further sub-divided into PSA-α1-antichymotrypsin (ACT) and PSA-α2-macroglobulin (2M). Free PSA (fPSA) accounts for the other 16% of tPSA and can be further sub-divided into the isoforms pro-PSA, benign PSA (BPSA) and inactive PSA (iPSA).
The unchallenged use of PSA for nearly two decades led to a large number of men who were in-fact suffering from indolent PCa undergoing unnecessary treatment leading to life-altering issues such as incontinence or impotence. Since PSA is not a cancer-specific marker and current clinical tests for serum PSA cannot distinguish between indolent and aggressive PCa, it should never have been used as a diagnostic test. Scientists proposing new biomarkers for PCa must critically assess whether the suggested biomarkers or diagnostic approaches meet all the necessary criteria (Table 1).
Table-1. Criteria for a Prostate Cancer Biomarker
| Criteria for a Prostate Cancer Biomarker |
|---|
|
1. Is cancer-specific. 2. Can detect the presence of disease.3. Distinguishes between indolent and aggressive prostate cancer. 4. Monitors disease progression.5. Predicts recurrence of disease. 6. Monitors response of disease to treatment.7. Is stable and can be easily detected. |
Prostate Cancer Staging
A number of factors are considered when assessing the significance of a man’s PCa. These include the age of the patient, the clinical stage of the cancer and the grade of the cancer. Combining this information with PSA levels allows clinicians to make informed decisions regarding optimal patient care options and patient prognosis (Figure 3). The stage of the cancer is determined using the Union for International Cancer Control (UICC) TNM (Tumour, Lymph Node, Metastasis) staging system19. The pathological grade of the cancer is determined post-biopsy using the Gleason Score which is an indicator of the aggressiveness of the cancer. Most tumours will be assigned a score between 6 and 10 where 6 is considered low grade and less aggressive and 10 is considered high grade and very aggressive20. Many PCas are slow growing and non-life threatening. Active surveillance or ‘watching and waiting’ is often an appropriate treatment, therefore, for older patient’s suffering from low grade tumours with a Gleason Score of 6. Following compilation of all available data, a patient will be assigned a clinical stage of PCa ranging from Stage I to Stage IV.
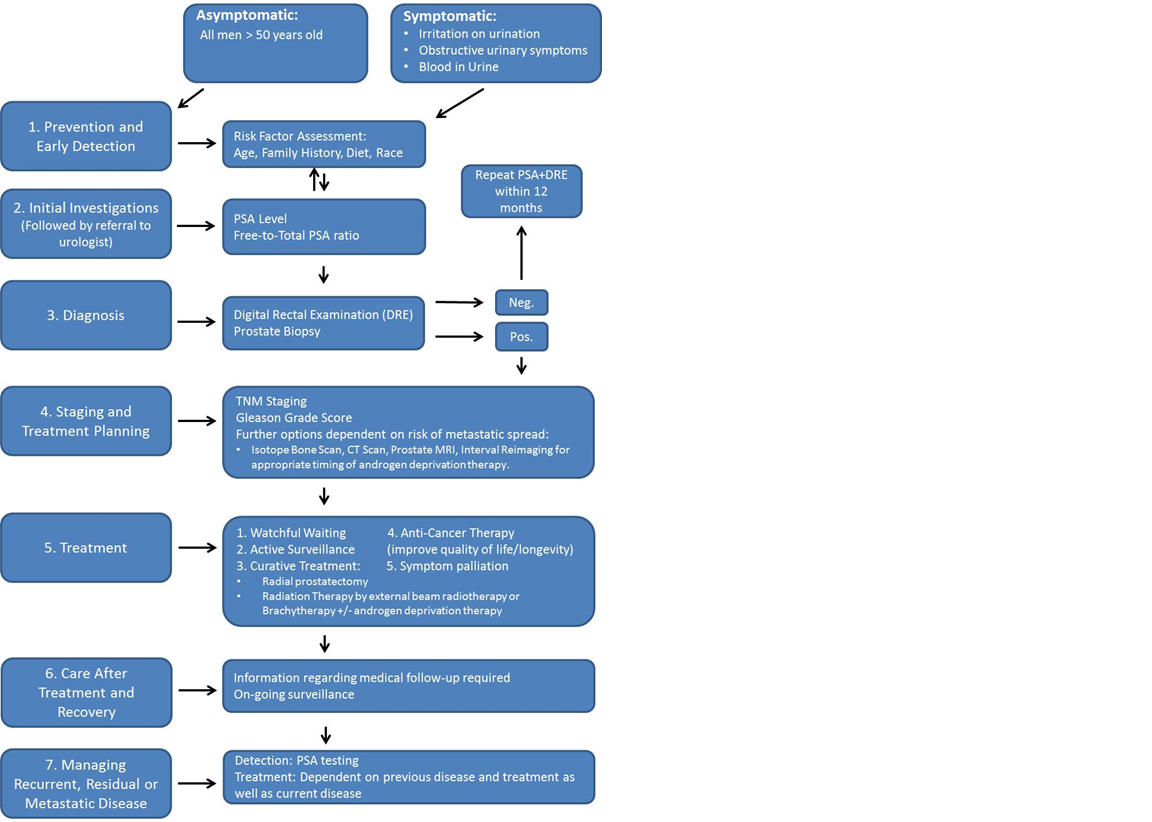
Figure 3: Optimal Care Pathway for men diagnosed with Prostate Cancer.
The Active Surveillance Era
Although PSA has made a comeback as a screening tool, it is now recommended for limited and cautious use18. To some extent it would appear that we have left the PSA era and moved into the Active Surveillance Era. This PCa management strategy, which was first introduced in the mid-90s, aims to prevent ‘quality-of-life’ reducing interventions to men with indolent PCa while still identifying aggressive, life-threatening PCa that could be addressed with rapid surgical and chemical intervention. It is currently the most rapidly growing approach to management of new PCa diagnoses with up to 40% of eligible men now choosing this treatment option. Epstein Criteria developed in 1994 are the most commonly used for entry into active surveillance. MRI-guided prostate biopsy is used for risk stratification and to detect disease progression21.
Commercially Available Diagnostic Tests
Although a move towards decentralised, patient-centred diagnostics is desired, the majority of testing remains in high-throughput, fully automated laboratories. Sharma and colleagues reviewed commonly used devices from 9 major manufacturers10. There was a difference of 18% in tPSA measurements and %fPSA levels varied between different assays22,23. This is a significant issue as it would affect clinical decisions relating to biopsy. Sharma et al. (2017) describe the motivation for introducing PSA testing as a way to ‘detect clinically significant PCa at an early stage’. This thinking, which resulted in the PSA era, ignored crucial scientific evidence which showed PSA cut-offs to be arbitrary and unable to diagnose PCa or identify clinically significant PCa24,19.
Consequently, the differential performance of the laboratory instruments is only important in terms of accuracy and clinical decision making regarding PCa recurrence, not diagnosis. PSA measurement should no longer be an infrequent event but a regularly monitored, quantitative event assessing rising PSA levels from the age of 50 onwards. For this reason, portable point-of-care devices ideally with the characteristics listed in Table 2 represent the most useful type of commercially available PSA test. Reproducibility and reliability are critical and currently, these devices are substandard when compared to the fully automated laboratory instruments25,10.
Table-2. Ideal characteristics of a Prostate Cancer Diagnostic Test
| Ideal Characteristics of a Prostate Cancer Diagnostic Test |
|---|
|
1. Accurate and sensitive detection of biomarkers. 2. Can test whole blood.3. Detects the presence of disease. 4. Produces quantitative results.5. Distinguishes between indolent and aggressive prostate cancer. 6. Can be miniaturised and portable for use as a point-of-care device. |
Next Generation diagnostic tools for Prostate Cancer
The controversies of the PSA era and the concomitant over-treatment of men highlighted the unsuitability of using a single biomarker for diagnosis and management of PCa. Utilising a panel of diverse biomarkers is the most appropriate method. Many novel tests have been reported since 2013 but despite very positive results, DRE, PSA testing and prostate biopsy remain the primary methods of PCa diagnosis. These newly reported tests require exhaustive clinical validation to inspire confidence in medical professionals to preferentially adopt their use in the clinic.
Proteomic and genomic technological advancements have uncovered countless novel markers. Sharma et al. (2017) reviewed 13 novel multifactorial assays10. These tests utilise an array of biomarkers including PSA isoforms, metabolites, kallikrein protein biomarkers, T2:ERG gene fusion and exosomal RNA alongside clinical data such as age, DRE and prior biopsy results from test specimens including serum, urine and tissue (Table 3). The Prostate Health Index (PHI) and ExoDx Prostate (IntelliScore) (EPI) were the most favourable of these novel tests as they did not require the patient to undergo a prior DRE or biopsy. PHI assesses serum PSA, fPSA and p2PSA. It is a prime example of how serum PSA can still be beneficial in clinical decision making when used with caution as recommended by the USPSTF and in a novel manner. PHI eliminated ~40% of total biopsies in two cohorts totalling 956 people and avoided invasive treatment for 21% of individuals with clinically insignificant cancer10. Additionally, PHI has undergone testing in a large, real-time, clinical study in an academic centre with 345 patients26 (Tososian et al, 2017). The impact of PHI testing has not been previously assessed in the clinical setting. It was determined that PHI testing resulted in a 9% reduction in the rate of prostate biopsy and that it had no impact on the proportion of biopsies detecting cancers of Gleason Grade 2 or greater. Therefore, if used routinely it would reduce the rate of unnecessary biopsies without risk to detection of higher grade cancers. EPI could discriminate high-grade from low-grade cancer and benign disease with an area under the receiver-operating characteristics curve (AUC) of 0.77 in a multi-centre clinical evaluation study with a training target population (255 men; median age = 62; median PSA = 5ng/ml) and an AUC of 0.73 in an independent validation study of 519 patients10.
Table-3. A sample of existing Next Generation Prostate Cancer Testsa
To aid with risk stratification following positive biopsy
| Test | Specimen | Biomarker | Requires | Suitable for | Validated | ||
|---|---|---|---|---|---|---|---|
| DRE | Biopsy | Diagnosis | Prognosis | ||||
| To aid decision making in relation to biopsy | |||||||
| The Prostate Health Index | Serum | PSA, fPSA, p2PSA | No | No | Yes | Yes | Extensively >15 studies including one large scale clinical evaluation. |
| ExoDx Prostate (Intelliscore) test | Urine | 3 biomarkers on exosomal RNA | No | No | yes | yes | Independently validated in 519 patients. |
| To aid with re-biopsy recommendations | |||||||
| Progensa PCA3 assay | Urine | PCA3 and PSA RNA | Yes | no | Yes | Yes | Extensively >9 studies |
| Confirm MDx assay | Tissue | Aberrant methylation in GSTP1, ACT and RASSF1 | No | Yes | Yes | Yes | Two validation studies (n=350, n=498) |
| Polaris | Tissue | 31 genes | no | Yes | Yes | Yes | Extensively 9 studies |
| Stockholm 3 | Plasma | Plasma protein biomarkers, 232 single-nucleotide polymorphisms and clinical variables | Yes | no | Yes | Yes | Extensively n = 56,000 |
aSharma, S., Zapatero-Rodriguez, J., O’Kennedy, R. Prostate cancer diagnostics: Clinical challenges and the ongoing need for disruptive and effective diagnostic tools. Biotechnology Advances. 2017; 35: 135-149
The most extensively validated recent study is the Stockholm 3 (STHLM3) study which is a prospective study with 56,000 participants. STHLM3 identifies aggressive PCa with a Gleason Score of at least 7 (Figure 4). STHLM3 was found to perform significantly better than PSA alone (AUC PSA alone 0.56; AUC STHLM3 0.74). The STHLM3 model could avoid ~44% of benign biopsies and could reduce the number of biopsies by ~32% without compromising the ability to diagnose aggressive PCa (Gleason Grade 7)27. STHLM3 is an example of the precise type of new diagnostic test needed to replace the currently existing methods of PCa diagnosis. It demonstrates the effectiveness of combining different types of biomarkers with clinical data.
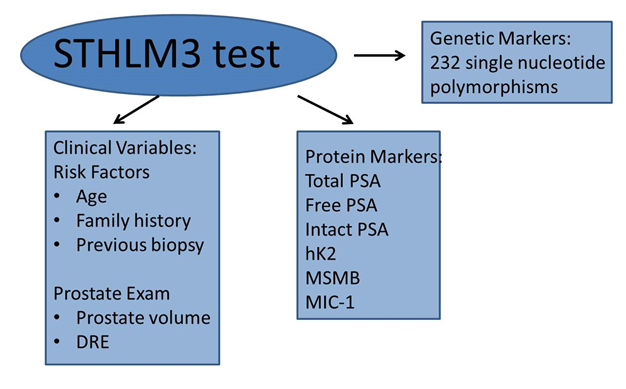
Figure 4: Stockholm 3 (STHLM3) test predicts aggressive prostate cancer.
The STHLM3 test combines clinical variables, protein markers and genetic markers to identify Gleason Grade 7 prostate cancer. DRE – Digital Rectal Examination; PSA – Prostate Specific Antigen; hK2 – human kallikrein 2; MSMB – microseminoprotein-beta; MIC-1 – macrophage inhibitory cytokine 1.
https://sthlm3.se/stockholm3-in-english/ [Internet]. [Accessed 16/10/2017].
Novel Pathways for Prostate Cancer Diagnostics
Autoantibodies, produced in response to tumour-associated antigens, are promising biomarkers due to their immediate presence following any perturbation to the cellular microenvironment of the prostate gland. They represent ideal candidates for early detection of PCa which is critical to ensure positive patient outcomes. Massoner et al. (2012) identified autoantibodies against 174 novel antigens exclusively present in the sera of 20 PCa patients28. In an independent cohort the autoantibody profile could discriminate between PCa patients and benign disease patients with an AUC of 0.71. Autoantibody profiles could represent a high-sensitivity test for early detection of PCa, superior to any existing test29.
Glycosylation is a very common post-translational modification that can be altered in cancer15. Llop et al. (2016) detected decreases in core fucosylation and an increase in the α-2,3-sialic acid percentage of PSA N-glycans in serum of PCa patients30. Both the PSA core fucose ratio and the percentage α-2,3-sialic acid could identify high-risk PCa patients. Significantly, Llop and colleagues reported that using a cut-off value of 30% for the α-2,3-sialic acid percentage of PSA, they could discriminate between the high-risk PCa patients and a group including benign prostatic hyperplasia, low- and intermediate-risk PCa. This was achieved with a sensitivity, specificity and AUC of 85.7%, 95.5% and 0.97, respectively. If translated into the clinic this finding could potentially revolutionise diagnosis and management of PCa by differentiating patients with indolent PCa from patients with aggressive PCa.
Conclusions
The key to effective PCa management is early, accurate diagnosis including the ability to differentiate between men with aggressive disease that require immediate treatment and those with slow-growing cancer that may not cause any significant difficulties within their lifetime. The capacity to make this distinction will save countless men from unnecessary treatment and facilitate rapid, appropriate, life-saving treatment for those that need it. There are many promising novel tests currently existing and if thoroughly validated in the clinical setting they could revolutionise PCa diagnosis.
Acknowledgments
This work was funded by Science Foundation Ireland (SFI) under Grant No.14/IA/2646.
Conflict of interests statement
Author Disclosure Statement: The authors declare no conflict of interest. No author or immediate family member has financial relationships with commercial organizations that might appear to represent a potential conflict of interest with the material presented.
References
- Denmeade SR, Isaacs, JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002; 2(5): 389-396.
- Lee DJ, Mallin K, Graves AJ, et al. Recent Changes in Prostate Cancer Screening Practices and Epidemiology. The Journal of Urology. 2017; 198:1-11.
- Centers for Disease Control and Prevention [Internet]. [June 5, 2017; accessed 26/09/2017]. Available from https://www.cdc.gov/cancer/dcpc/data/men.htm.
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. European Journal of Cancer. 2013;49: 1374-1403.
- Center MM, Jemal A, Lortet-Tieulent J, et al. International Variation in Prostate Cancer Incidence and Mortality Rates. European Urology. 2012; 61:1079-1092.
- GLOBOCAN 2012 (IARC): Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 [Internet] Accessed 03/10/2014. Available from: www.globocan.iarc.fr
- Harvey CJ, Pilcher J, Richenberg J, et al. Applications of transrectal ultrasound in prostate cancer. British Journal of Radiology. 2012; 85:S3-S17.
- Friedman GD, Hiatt A, Quesenberry Jr P, et al. Case-control study of screening for prostatic cancer by digital rectal examinations. The Lancet. 1991; 337(8756): 1526-1529.
- Richert-Boe KE, Humphrey LL, Glass AG, et al. Screening digital rectal examination and prostate cancer mortality: a case-control study. J Med Screen. 1998; 5: 99-103.
- Sharma S, Zapatero-Rodriquez J, O’Kennedy R. Prostate cancer diagnostics: Clinical Challenges and the ongoing need for disruptive and effective diagnostic tools. Biotechnology Advances. 2017; 35: 135-149.
- Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008; 8: 268-78.
- Albin RJ, Piana R. The Great Prostate Hoax: How Big Medicine Hijacked the PSA Test and Caused a Public Health Disaster. United States: Palgrave McMillian; 2014.
- Albin RJ. New Scientist [Internet]. Magazine Issue 2956, 15th February 2014. [Accessed 16/10/2017]. Available from: https://www.newscientist.com/article/mg22129564-400-prostate-cancer-test-has-been-misused-for-money/
- Saini S. PSA and beyond: alternative prostate cancer biomarkers. Cell Oncol Dordr. 2016; 39(2):97-106.
- Gilgunn S, Conroy PJ, Saldova R, et al. Aberrant PSA glycosylation--a sweet predictor of prostate cancer. Nat Rev Urol. 2013; 10: 99-107.
- Moyer VA, Force USPSTF. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 157: 120-34.
- Fleshner K, Carlsson SV, Roobol MJ. The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat Rev Urol. 2017; 14: 26–37.
- Van der Kwast TH, Roobol, MJ. Prostate cancer: Draft USPSTF 2017 recommendation on PSA testing — a sea-change. Nat Rev Urol. 2017; 14(8): 457-458.
- Solyanik O, Schlenker B, Gratzke C, et al. Imaging of the locally advanced prostate carcinoma. The Urologist. 2017. https://doi-org.dcu.idm.oclc.org/10.1007/s00120-017-0515-0
- American Cancer Soceity [Internet]. [Accessed 16/10/2017]. Available from www.cancer.org
- Elkhoury FF, Simopoulos DN, Marks LS. Targetted Prostate Biopsy in the Era of Active Surveillance. Urology. 2017. https://doi.org/doi:10.1016/j.urology.2017.09.007
- Stephan C, Klass M, Muller, C. Interchangeability of measurements of total and free prostate-specific antigen in serum with 5 frequently used assay combinations: an update. Clin. Chem. 2006a; 52: 59-64.
- Stephan C, Klass M, Muller C, et al. Interchangeability of measurements of total and free prostate-specific antigen in serum with 5 frequently used assay combinations: an update. Clin Chem. 2006b; 52: 59-64.
- Clements R. Prostate-specific antigen: an opinion on its value to the radiologist. Eur Radiol. 1999; 9: 529-535.
- Rausch S, Hennenlotter J, Wiesenreiter J, et al. Assessment of a new point-of-care system for detection of prostate specific antigen. BMC Urology. 2016; 16: 4.
- Tosoian JJ, Druskin SC, Andreas D, et al. Use of the Prostate Health Index for the Detection of Prostate Cancer: Results from a Large Academic Practice. Prostate Cancer Prostatic Dis. 2017; 20(2): 228-233.
- Grönberg H, Adolfsson J, Aly M, et al. Prostate cancer screening in men aged 50 to 69 years (STHLM3): A prospective population-based diagnostic study. Lancet Oncol. 2015; 16(16): 1667-76.
- Massoner P, Lueking A, Goehler H., et al. Serum-autoantibodies for discovery of prostate cancer specific biomarkers. Prostate. 2012; 72(4): 427-36.
- Schipper M, Wang G, Giles N, et al. Novel prostate cancer biomarkers derived from autoantibody signatures. Transl Oncol. 2015; 8:106-111.
- Llop E, Ferrer-Batalle M, Barrabes S, et al. Improvement of Prostate Cancer Diagnosis by Detecting PSA Glycosylation-Specific Changes. Theranostics. 2016; 6(8): 1190-1204.
