Mini-Review: Pancreatic Cancer Presenting as a Bowel Obstruction and the Role of Next-Generation Sequencing
Bach Ardalan1*, Jose Azqueta1, Jonathan England1, Danny Sleeman1, Rene Hartmann2
1Sylvester Comprehensive Cancer Center
2Baptist Hospital of Miami
Abstract
Pancreatic adenocarcinoma has increasingly become one of the leading causes of death in western countries. However, presentation with colonic metastases is far less frequently reported in the literature and may be misdiagnosed as colonic adenocarcinoma. This is a case of a female patient with metastatic pancreatic adenocarcinoma that presented with a sigmoid obstruction.
Case Description
The case described is a 66-year-old Hispanic female who presented to a local hospital complaining of abdominal pain and constipation. She was found to have an obstructing sigmoid lesion and underwent an emergency left hemicolectomy and ostomy creation. Surgical pathology indicated adenocarcinoma (Figure 1). Liver metastases and a pancreatic tail lesion were seen, and the hepatic metastases were biopsied. Pathology revealed adenocarcinoma of pancreatobiliary origin (Figure 2). She was treated with two attenuated cycles of FOLFIRINOX, reportedly well tolerated.
She transferred her care to the United States at Sylvester Cancer Center. Her pathology slides were reviewed and were found to be adenocarcinoma of pancreatobiliary origin [CK7 positive, and CK20 negative]. New imaging studies were requested indicating pancreatic tail mass, hepatic metastases, and multiple omental lesions, notably one adjacent to her stoma (Figures 3 through 5). A liquid biopsy was performed at baseline, indicating BRCA D1923N, KRAS G12R mutation, and FGFR1 amplification. Her case was discussed at tumor board. She was given one cycle of FOLFIRINOX, with poor toleration. Her clinical status continued to deteriorate, and the patient elected to stop chemotherapy and seek hospice care.
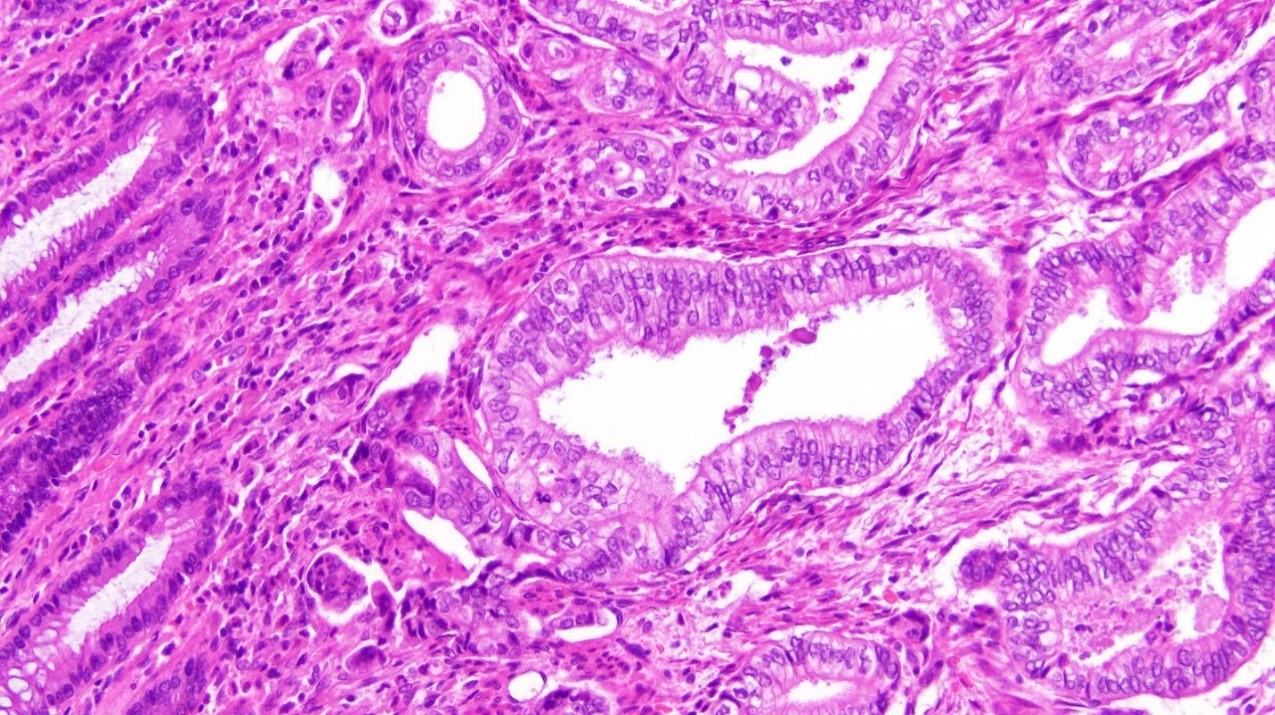
Figure 1: Adenocarcinoma found in sigmoid colon (x20 magnification)
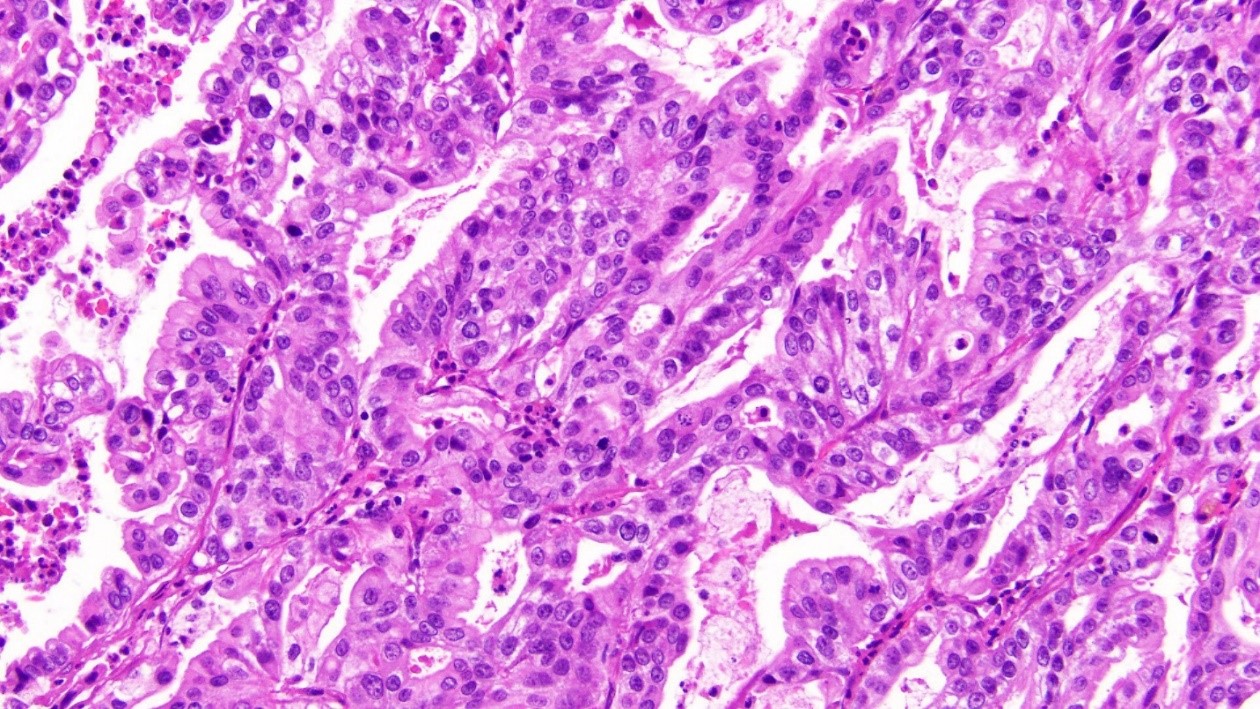
Figure 2: Adenocarcinoma found within hepatic parenchyma (x20 magnification)
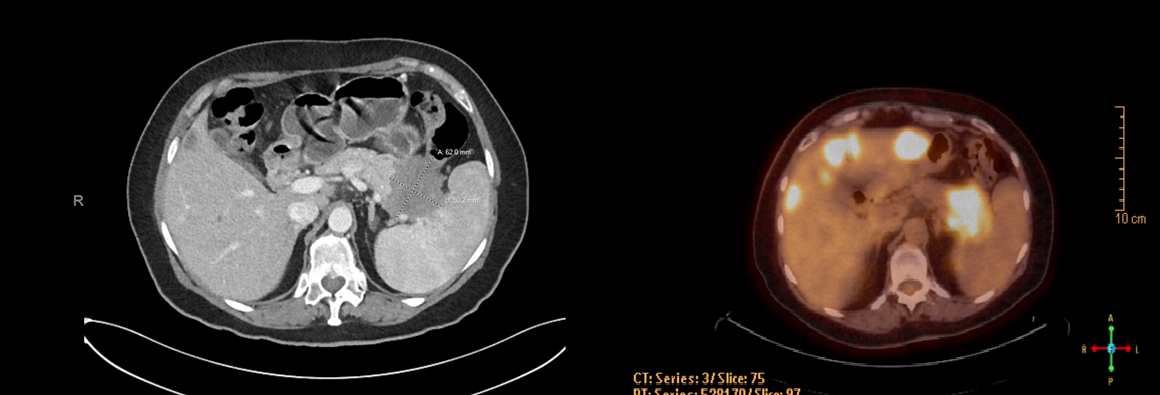
Figure 3: Pancreatic Tail lesion (CT with contrast and PET/CT, side by side). The prominent pancreatic tail mass invades into the splenic hilum, stomach, and splenic flexure, encasing the splenic vessels
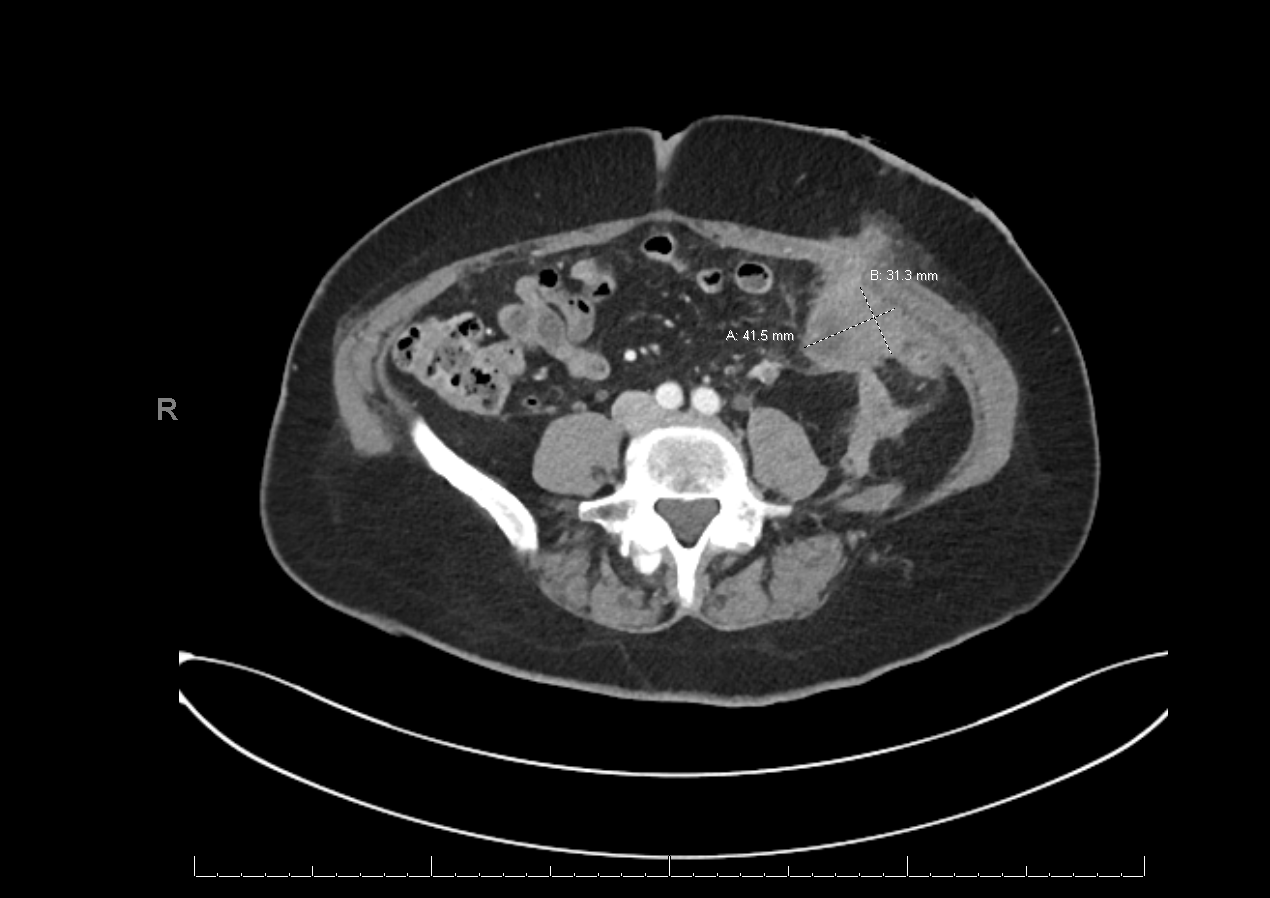
Figure 4: Prominent omental lesion adjacent to the site of stoma
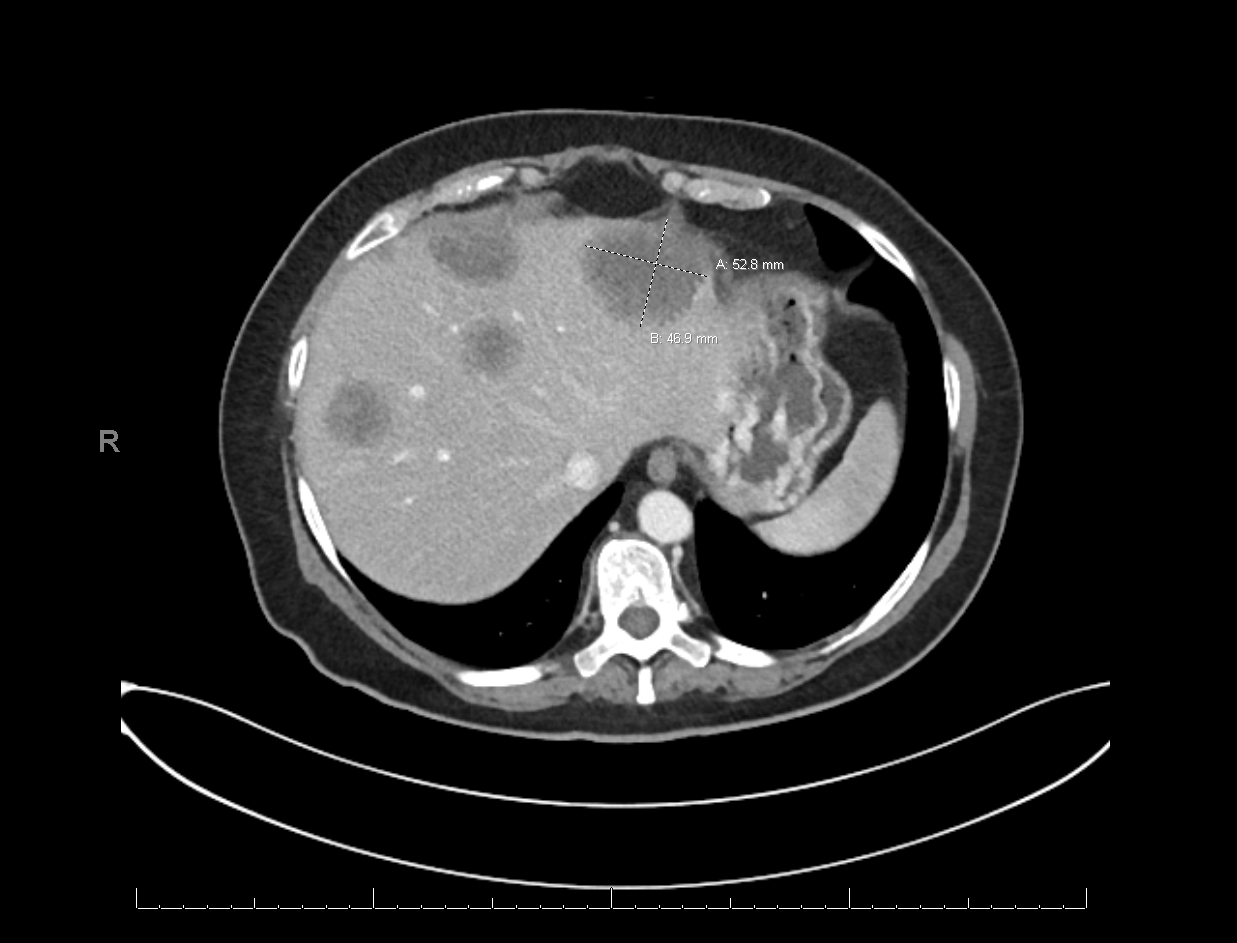
Figure 5: Multiple hepatic metastases, biopsy confirmed to be of pancreatobiliary primary
Background
Pancreatic cancer is one of the more increasingly common solid tumor malignancies in western countries with an overall survival of 9-10% at 5 years1. It is often diagnosed at later stages, at which point the disease has metastasized to distant sites, typically to the liver. Colonic metastases is rarely reported in the literature, possibly misdiagnosed as adenocarcinoma of the colon. In pancreatic cancer, about 95% of the tumors are KRAS mutated, on codon 122.
In solid tumors, about 25-30% are found to have RAS mutations, with KRAS being the most frequently mutated isoform3. Although rare in other types of solid tumors, KRAS G12R is commonly found in pancreatic cancer as high as 15-20%4.
As the rate of pancreatic cancer has steadily increased over the past decade, so has its presentations with colonic metastases. Synchronous presentation of pancreatic cancer with colonic metastases are rare, with only a few reported cases in the literature to date.
The first reported case in the literature of synchronous metastases to the colon presented as an ascending colon lesion. The colonic mass was resected and determined to be of pancreatic origin. No further follow up was given5.
The second case reported in the literature of synchronous colonic metastases of a pancreatic primary is that of a 67-year-old female with worsening abdominal pain and constipation. At presentation, she was found to have a lesion on the distal sigmoid colon, peritoneal nodules and mass at the tail of the pancreas. She underwent resection of the peritoneal implants and colectomy with the creation of an end colostomy. Although the patient was initiated on FOLFIRINOX chemotherapy, she did not tolerate the first round and thereafter was discharged home to hospice6.
The third reported case in the literature describes a 60-year-old male who presented with thickening of the sigmoid colon, pancreatic tail mass involving the spleen and left kidney, multiple mesenteric masses, suggesting metastatic disease. Biopsy of an umbilical nodule revealed metastases from pancreatic adenocarcinoma. The patient underwent palliative FOLFOX chemotherapy; however, his colonic lesion perforated. Thereafter, the patient was admitted for emergency surgery. He continued to receive palliative treatment and died 9 months after his diagnosis7.
The fourth reported case reported a 91-year-old patient who presented with 13.6kg weight loss and a long-reported history of constipation. She underwent sigmoidoscopy; however, stenosis was found in the sigmoid colon and it was unable to be traversed. She received palliative sigmoid colectomy with end colostomy. Thereafter, a CT scan was performed revealing a pancreatic head mass. Pathology of the metastatic lesion revealed well-differentiated adenocarcinoma with CK7 positive and CK20 negative staining. CA19-9 was elevated. Patient desired comfort care and was discharged home with hospice care8.
The fifth reported case in the literature was that of a 71-year-old male with synchronous descending colon/sigmoid lesion, with elevated CA19-9. The lesion was biopsied and immunohistochemistry showed positive CK7 staining and only few cells of CK20, thereby confirming pancreatic origin. Thereafter, the patient initiated palliative abraxane/gemzar chemotherapy. No further follow up was reported9.
The final reported case in the literature is that of a 74-year-old male patient who presented to his local physician with tarry stools and significant lethargy. Baseline imaging was noncontributory, and the patient was admitted. Upon admission the patient underwent colonoscopy which revealed a lesion at approximately 70 cm from anal verge. Mass prevented further examination, and patient underwent left hemicolectomy, splenectomy and partial distal pancreatectomy. Pathology demonstrated pancreatic ductal adenocarcinoma with extension into wall of the splenic flexure. It was noted that the patient’s CA19-9 was elevated, and the carcinoma invaded the peripancreatic soft tissue10.
Discussion
In our patient, a liquid biopsy was performed in order to determine any targetable mutations. Among the ones reported, her tumor harbored KRAS G12R mutation. The KRAS oncogene is involved in the MAPK/ERK pathway and participates in cell differentiation, proliferation, and cell death. KRAS G12R is commonly found in pancreatic cancer, totaling 15% overall in comparison to the more frequent G12D and G12V mutations4. Furthermore, patients who have this mutation tend to have a better overall prognosis in comparison to G12D11. In our previous studies, KRAS G12R mutated patients have shown benefit when MEK inhibitors were included in the chemotherapy12,13. Unfortunately, baseline next-generation sequencing may take one to three months, hence causing a delay in the targeted approach in the treatment of our pancreatic cancer patients.
The majority of pancreatic cancer patients are G12D mutated with a frequency of 51%, followed by G12V (30%), G12R (15%), and G12C/S (2% each). Less frequently occurring mutations are observed on codon 13 (i.e., G13C/D/P/S) and codon 61 (Q61H/K/R). Multiple attempts in the past decade have been tried to target the RAS pathway in pancreatic cancer, however as of yet there have been no sucesses13. The agents used have shown inability to bind to the small binding pockets of KRAS, and the high guanine triphosphate concentration has rendered KRAS protein undruggable.
Recently, the novel compound sotorasib has shown promise in KRAS G12C lung tumor by binding to the His95 groove causing high levels of inactive KRAS14. In pancreatic adenocarcinoma, KRAS G12C mutation occurs in less than 2% of patients in comparison to KRAS G12R, which is commonly seen in pancreatic adenocarcinoma at the rate of 15%. The phosphoinositide 3-kinases (PI3k)/phosphatase and tensin homolog (PTEN)/protein kinase B (Akt)/mammalian/mechanistic target of rapamycin complex 1 (mTORC1) are key pathways within RAS mutated pancreatic cancer. Monotherapy targeting of PI3k, AKT, and mTOR has not been successful in RAS-mutated pancreatic cancers. Dual PI3K in combination with RAF-MEK-ERK inhibitors is currently being tested and hold promise given G12R operating via PI3K gamma (Figure 6)15. A randomized phase II study of the MEK inhibitor, selumetinib and MK-2206, an AKT inhibitor, failed to show any benefit when compared to the cohort of patients treated with FOLFOX, who had previously failed gemcitabine-based therapy16,17. It should be noted that these agents were given as a monotherapy.
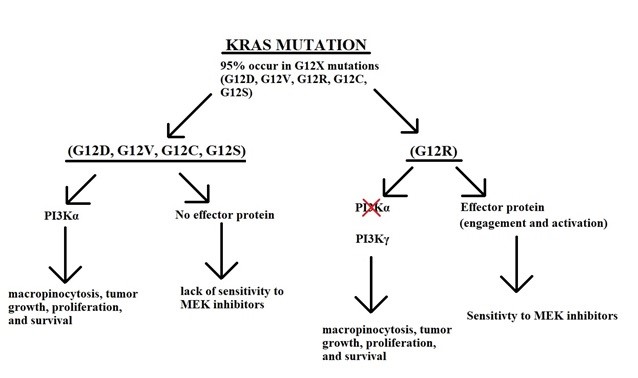
Figure 6: KRAS pathways. All the common mutations G12D,G12V,G12C and G12S, operate through PI3K alpha, and have NO effector protein to interact with the MEK inhibitors. On the other hand G12R operates via PI3K gamma and HAS an effector protein which interacts with MEK inhibitor. This interaction is augmented in the presence of gemcitabine.
In the past, several attempts have been made to incorporate various MEK inhibitors into clinical practice. In one randomized double-blinded placebo-controlled trial of trametinib in combination with gemcitabine in patients with pancreatic cancer who were previously untreated, the study was negative with no significant overall survival favoring trametinib18. However, there were several inconsistencies within the study. Firstly, in pancreatic adenocarcinoma typically less than 5% of patients are KRAS wild type, and 95% are KRAS mutated; in this study however, the KRAS wild type was reported to be over 20%. Secondly, in the group of KRAS wildtype patients, there was no mention of other mutations within this cohort, such as BRAF V600E. Thirdly, the authors did not delineate the subgroups of KRAS mutations within their study. The authors reported fifty patients with KRAS mutations which would translate to seven patients with KRAS G12R on each arm18. This number is based on the frequency of G12R patients in the KRAS-mutated pancreatic cancer patient population4.
In support of our hypothesis, a recent study of the MEK inhibitor selumetinib, was administered as a single agent in heavily pre-treated pancreatic cancer patients. All patients were advanced pancreatic cancer patients harboring a KRAS G12R mutation, who have previously failed a prior line of therapy. After a follow up period of 8.5 months, three patients were found to have stable disease; median overall survival was 9 months in this cohort, and median progression free survival was 3 months19.
Conclusion
Metastases from pancreatic adenocarcinoma to the sigmoid colon is rare, but it has been noted in the literature. Although our patient was not well enough for further chemotherapy, she may have benefited from the possible combination of chemotherapy and a MEK inhibitor given her KRAS G12R mutation. In addition, whole genome analyses has allowed for the development of targeted therapies, indicating added benefit of baseline molecular sequencing of the patient’s tumor. We believe it is pertinent for oncologists to order next-generation sequencing on the patient’s tumor at the time of the initial visit since typically the result of the tests are not available for several months.
References:
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: a cancer journal for clinicians. 2020 Jan 1; 70(1): 7-30.
- Haigis KM. KRAS alleles: the devil is in the detail. Trends in cancer. 2017 Oct 1; 3(10): 686-97.
- Cox AD, Fesik SW, Kimmelman AC, et al. Drugging the undruggable RAS: mission possible? Nature reviews Drug discovery. 2014 Nov; 13(11): 828-51.
- Pantsar T, Rissanen S, Dauch D, et al. Assessment of mutation probabilities of KRAS G12 missense mutants and their long-timescale dynamics by atomistic molecular simulations and Markov state modeling. PLoS computational biology. 2018 Sep 10; 14(9): e1006458.
- Bellows C, Gage T, Stark M, et al. Metastatic pancreatic carcinoma presenting as colon carcinoma. Southern medical journal. 2009 Jul 1; 102(7): 748-50.
- Kelley KM, Myer BS, Berger JJ. Malignant large bowel obstruction: a rare presentation of metastatic pancreatic cancer. The American Surgeon. 2016 Aug; 82(8): 206-8.
- Nogueira ST, Pinto BL, Silva EF, et al. Pancreatic cancer presenting as colonic disease. A rare case report. International Journal of Surgery Case Reports. 2018 Jan 1; 44: 4-7.
- Ryan Kahl KG, Patel K, Stawick L. Pancreatic adenocarcinoma with rare sigmoid colon metastasis. ACG Case Reports Journal. 2019 Jul; 6(7)
- Yewale R, Ramakrishna B, Vijaykumar K, et al. Pancreatic adenocarcinoma with synchronous colonic metastases. ACG Case Reports Journal. 2020 Jan; 7(1).
- Ekanayake LS, Jenkins D, Swade S, et al. Locally invasive pancreatic adenocarcinoma presenting in the splenic flexure: a rare case report. Cureus. 2020 May 13; 12(5).
- Bournet B, Muscari F, Buscail C, et al. KRAS G12D mutation subtype is a prognostic factor for advanced pancreatic adenocarcinoma. Clinical and translational gastroenterology. 2016 Mar; 7(3): e157.
- Ardalan B, Azqueta J, Sleeman D. Cobimetinib Plus Gemcitabine: An Active Combination in KRAS G12R-Mutated Pancreatic Ductal Adenocarcinoma Patients in Previously Treated and Failed Multiple Chemotherapies. Journal of Pancreatic Cancer. 2021 Oct 1; 7(1): 65-70.
- Gillson J, Ramaswamy Y, Singh G, et al. Small molecule KRAS inhibitors: the future for targeted pancreatic cancer therapy? Cancers. 2020 May; 12(5): 1341.
- Canon J, Rex K, Saiki AY, et al. The clinical KRAS (G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019 Nov; 575(7781): 217-23.
- Hobbs GA, Baker NM, Miermont AM, et al. Atypical KRASG12R mutant is impaired in PI3K signaling and macropinocytosis in pancreatic cancer. Cancer discovery. 2020 Jan 1; 10(1): 104-23.
- Saini KS, Loi S, de Azambuja E, et al. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer treatment reviews. 2013 Dec 1; 39(8): 935-46.
- Chung V, McDonough S, Philip PA, et al. Effect of selumetinib and MK-2206 vs oxaliplatin and fluorouracil in patients with metastatic pancreatic cancer after prior therapy: SWOG S1115 study randomized clinical trial. JAMA oncology. 2017 Apr 1; 3(4): 516-22.
- Infante JR, Somer BG, Park JO, et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. European journal of cancer. 2014 Aug 1; 50(12): 2072-81.
- Kenney C, Kunst T, Webb S, et al. Phase II study of selumetinib, an orally active inhibitor of MEK1 and MEK2 kinases, in KRASG12R-mutant pancreatic ductal adenocarcinoma. Investigational new drugs. 2021 Jun;39(3):821-8.
