Potential Transdermal Cancer Treatment Through Blood Brain Barrier with a Crystalline Neratinib Salt
Thomas E. Beesley1* and Mark V. Beesley2
1TEB Chiral View LLC, Consulting, Towaco, NJ 07082, USA
2MB Manufacturing, Blairstown, NJ 07825, USA
Abstract
The tyrosine kinase inhibitor Neratinib has demonstrated its efficacy against HER2+ breast cancer metastases as the maleate salt. It has, however, had only a limited success as the maleate acid salt to cross the blood-brain barrier (BBB) due in part to its low oral absorption rate. At high oral doses severe diarrhea occurs. The potential to treat metastasized HER2+ cancer in the cerebellum and spinal cord that effects 30-50% of patients with this condition with a new crystalline salt and a topical application of a dimethyl sulfoxide (DMSO) solution has been investigated. This work proposes to use different Neratinib salts to improve solubility and BBB transport by a transdermal rather than an oral administration protocol. The preparation of the salts is described, and analytical methods developed for LC and LC/MS. The log P coefficient utilizing octanol/buffered water, shake flask method, will be used as a predictor of potential improvement in BBB transfer due to increased lipophilicity. Solubility data is supplied. An extensive review of DMSO and BBB experiments was undertaken leading to the establishment of rules to make this methodology work. First, a log P in the range of 2-4 is essential. Second, amine functions have to be blocked with suitable salts. If an amine function is present, it will be trapped by the endothelial membrane. DMSO has a well-documented favorable toxicological profile and can be sterilized making it safe for oral and parenteral routes. A transdermal protocol using Neratinib succinate in 20/20/60: H2O/EtOH/DMSO is described with all the safety measures presented.
Introduction
The focus of this investigation has been patients diagnosed with stage 4 metastatic human epidermal growth factor receptor 2 positive (HER2+) cancer, specifically when the metastasized cancer had progressed to the cerebellum and spinal cord. Leptomeningeal disease affecting the intracranial cavity containing the brain stem and cerebellum occurs in 30-50% of patients with metastatic HER2+ breast cancer according to a 2012 report.1 Neratinib as an oral medication had demonstrated success against HER2+ type of cancer as the maleic acid salt.2 Due to its molecule size, structure and solubility, however, Neratinib maleate could not sufficiently cross the blood-brain barrier (BBB) to reach those affected areas even with a log P of 3.16. It was reported that a small group of 40 patients demonstrated a very limited response in the brain area.3 Phase 2 and 3 trials in the United States and phase 4 trials in Australia have indicated a greater success rate for Neratinib maleate to contain and reverse metastasized HER2+ cancers.4,5 Used as an oral medication in very high doses, diarrhea was indicated as the only major side effect which was somewhat controlled with Imodium.6 The forgoing set of circumstances indicates that if you could bypass the digestive system and direct the drug to the affected area you could positively treat this difficult situation.
Of all the methods used to disrupt the BBB; neurosurgical, radiation or chemical, dimethyl sulfoxide (DMSO) has been shown to be the most effective and least destructive methodology. In addition, DMSO has a well-documented favorable toxicological profile. The oral LD 50 in rats is 17.9-28.3 mg/kg indicating very low toxicity.7 It has been known for some time that DMSO can effectively transmit a number of drugs topically to a targeted organ.8 It has been further demonstrated that DMSO can alter the BBB to allow the topically applied drugs to reach the affected areas beyond the BBB and do so quickly and safely.9 We were further encouraged by the fact that DMSO was reported to lower intracranial pressure reducing swelling and lowering pressure after closed head trauma.10,11 This is a beneficial effect since it has been noted that cerebral fluid becomes increasingly viscous with cancer invasion. This indicates that DMSO has beneficial effects when exposed to brain tissue, a necessary safeguard.
With the expanded use of DMSO in pharmaceutical applications as reported in the 2008 issue of Pharmaceutical Technology12, more attention should be paid to this approach to treat this difficult situation with metastasized cancer to the CNS. To further establish the effectiveness of the DMSO approach as a topically applied method, a 45% aqueous solution of DMSO was used to administer Diclofenac (MW 296.15) to a torn calf muscle by this investigator and the pain relief occurred in 30 seconds.13 This experiment and the related literature demonstrated the speed of transport of a drug in DMSO through the stratum corneum. While concentrations up to 100% DMSO had been shown to have the greatest effect on drug adsorption, damage to cell structure became apparent at this high concentration after repeated applications. Considering that DMSO is fully hydrated at 67% weight strength in water, a 60 % solution was determined to be a good, safe compromise and starting point to allow for good permeability and solubility with no permanent alteration of the skin surface or capillary structure. Dependent on the response under those conditions a concentration of up to 80% DMSO may be necessary in future investigations which would still not permanently affect the cell structure.
Factors that normally affect the skin adsorption of a drug include: concentration, molecular weight, duration of contact, solubility and physical condition of the skin and the part of the body to be exposed. There is a hierarchy for various parts of the body that are most affected by skin adsorption.14 Typically, there is an ~ 500 Dalton rule for skin adsorption that has been determined using either dry contact or an aqueous solution of the compound.15 We are suggesting the use of DMSO as a transport system to avoid metabolism of the Neratinib in the gut by applying it directly over the affected spinal cord and the base of the brain, a mid-sensitive area.
There are two problems with Neratinib that have reduce its effectiveness for treating cancers that have metastasized to the central nervous system. First is the lack of solubility and lipophilicity because it is a basic compound. Second was the need for appropriate contact time on the skin surface to allow for alteration of the stratum corneum structure allowing for permeability of a proper Neratinib salt. To address the first case, we referred to U.S. Patent 9139558 B2.16 In this study, several salts of Neratinib were investigated for the purpose of increasing its bioavailability. The chosen salt also increased Neratinib’s solubility, both important factors if it is going to be effective for this application. Neratinib is soluble in a variety of polar solvents but has limited water solubility. We reevaluated the salts in the Wyeth patent because the one that was chosen was the one useful salt they claimed they were able to crystallize but to us the succinate or the glutarate should have been a more neutral, suitable structure to cross the BBB.
Neratinib being slightly over the 500 Dalton range (557.02) the effect could possibly be ameliorated with the proper concentration of DMSO. It should also be noted that DMSO has analgesic, anti-inflammatory and cryoprotective properties.17 The only FDA approved application of DMSO in the 1970’s was for the treatment of interstitial cystitis. A review in Pharmaceutical Technology has cited an increasing number of approved applications for DMSO. These included delivery of medical polymers, as a solubilizing agents in prostate cancer treatment, in sustained release applications, transdermal applications and as an active pharmaceutical ingredient.12 Repeated topical applications may result in mild scaling dermatitis but the methodology appears quite safe. There is substantial evidence that DMSO can increase diffusion through the stratum corneum by disruption of the barrier function. This probably occurs through aprotic interactions with cellular lipids and may also reverse distortion of lipid head groups. The current status of DMSO research for drug delivery is reviewed18.
Possible Rules for Crossing the Blood-Brain Barrier
A very comprehensive report in NeuroRx by William Pardridge outlines perfectly the failures of ignoring brain drug development since the BBB excludes from the brain 100% of large molecule neurotherapeutics and more than 98% of all small molecule drugs.19 This article reports on three possibilities in getting drugs pass the BBB and the benefits and drawbacks of the three methodologies. The first is direct intracerebroventricular infusion that results in minimal penetration into the brain due to a lack of a lymphatic system to aid in the perfusion process. Second is to take advantage of the highly stereospecific pore-based transport system. An example cited was the α-carboxylation of dopamine to L-DOPA ( MW 197.19). Once the large neutral amino acid crosses the BBB the L-DOPA is decarboxylated to dopamine. This is a prime example of carrier mediation rather than lipid mediation.20 The final methodology is BBB disruption created by chemicals such as ethanol, dimethyl sulfoxide or surfactants like Tween 80, or sodium dodecyl sulfate (SDS). From a review of the above literature, of all of the solvent mediated disruption with chemicals, DMSO has shown the most success with the least damaging effects and the most quickly reversible effects on the BBB.
The endothelial cells forming the blood-brain barrier are highly specialized to allow precise control over substances that enter or leave the brain. Diffusion is the major entry mechanism for most psychoactive drugs and the rate of diffusion depends on the lipid solubility of the drugs. Drugs can be synthesized with high BBB permeability to improve entry into the brain. A typical example would be the acetylation of morphine to make heroin a more lipid soluble compound.21 Morphine with a log P (0.99) when acetylated to Heroine log P (2.3) brain uptake goes from 2% to 70%. From a review of the literature a log P range of (2-4) seems most successful.22 Published failures also make the point. Methotrexate with a log P (-1.85) fails to cross the BBB with concentrations of DMSO from 20-90%.23 A number of examples have shown that amine functions specifically seem to interact with the endothelial membrane and have to be blocked either through an acid salt formation or acetylation. Morphine sulfate (M.W. 668.76) was transported to the brain by transdermal application in 90% DMSO.17 So the rule limit of <500 Daltons can be overridden with the use of the proper concentration of DMSO. Horseradish peroxidase (44K Daltons) can also be transferred only with 10 – 15% DMSO.24
Neratinib, a basic compound, when treated with a diacid to form a salt to block two amino groups increases its solubility at a physiologic pH. It also increases log P and shows promise in crossing the BBB. The ethanol crystallized succinate in this paper presents an unusual situation in that the ethanol seems to strongly bind to one amine site leaving one acid group of the diacid free changing the solubility and log P dramatically in the right direction.
Materials and Methods
Chemicals
All acids and chemicals used in the preparation of the salts were obtained from Millipore Sigma, St Louis, MO. DMSO from this source, although sterile filtered, is not suitable for healthcare applications. Gaylord Chemical Company, LLC Tuscaloosa, AL manufactures a USP, PH.EUR grade safe for oral and parenteral routes. DMSO, USP is reference in the Handbook of Pharmaceutical Excipients. JT Baker solvents were obtained from Seidler Chemical Company, Newark, NJ. The JT Baker solvents; water and methanol were HPLC certified and used as received. Pure Neratinib C30H29ClN6O3, M.W. 557, (structure 1) was obtained from Med Chem Express, Monmouth Junction, NJ and verified by LC/MS and carbon, hydrogen and nitrogen elemental analysis.
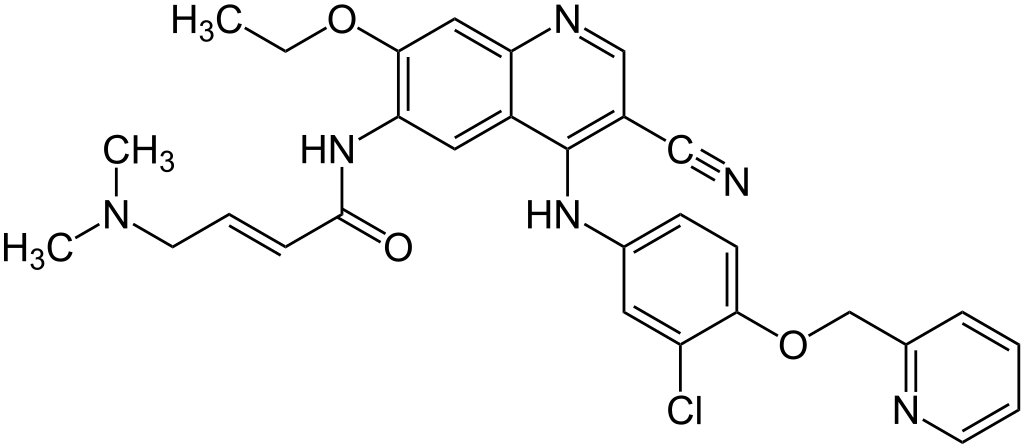
Structure 1: Neratinib, (3E)-N-[4-[3-chloro-4-[pyridine-2-yl)methoxy]-3-cyano-7-ethoxyquinolin-6-yl]-4-(dimethylamino)but-2-enamide, M.W. 557, pka = 7.65 and 4.6
Materials
Thin layer chromatography 20 x 20 cm plates, Whatman LK5DF prepared with a 254nm fluorescent material in an 80 A silica were obtained from Millipore Sigma, St. Louis, MO. High performance liquid chromatography was performed with a Shimadzu LC-40 system with dual pumps, UV detector and column temperature controller (Shimadzu, Kyoto, Japan) operated by Lab Solutions software (Shimadzu). A 150 x 4.6 mm Shim-Pak C18 column (Shimadzu) packed on 5 µm fully porous silica particles with a 19% carbon load was used for all analyses. The column was operated at 40°C.
Methods
All procedures were run multiple times in order to demonstrate good stability and reproducibility. Certified Neratinib was used as an internal standard in all our chromatographic procedures. We have developed an HPLC method that is robust and can be used for both standard HPLC as well as LC/MS. See Figure 2.
Preparation of Neratinib salts
Neratinib free base solutions (100 ml) were prepared dissolving 1 g (1.795 mM) Neratinib in 70 mL warm distilled water and diluted to 100 mL with warm absolute ethanol EtOH). The recorded pH was 7.9. The salts of maleic, succinic, glutaric, and l-malic acids were prepared similarly. Diacid (~1.8 mM) were added to 100 mL Neratinib warm solution (50-55°C) and agitated for few minutes giving a clear yellow solution with a pH ~4.5. Solution was then evaporated to dryness and the solid residue was redissolved in 50°C absolute ethanol. Upon cooling, the salt precipitated as fine needles and the excess acid stayed in cold ethanolic solution. The succinate salt also crystalized well from isopropyl alcohol (IPA). The crystalline solids were filtered off under vacuum. Table 1 presents the results. All salts were submitted for C, H, N elemental analysis returning respective values within less than 0.5%, 0.1%, and 0.2% of the theoretical values. TLC was used to check the salt purity. Fig. 1 illustrates the Neratinib maleate separation compared to pure Neratinib. Since TLC is not accurate enough to give concentrations, the salt purity and especially concentration were obtained by HPLC as shown by Fig. 2.
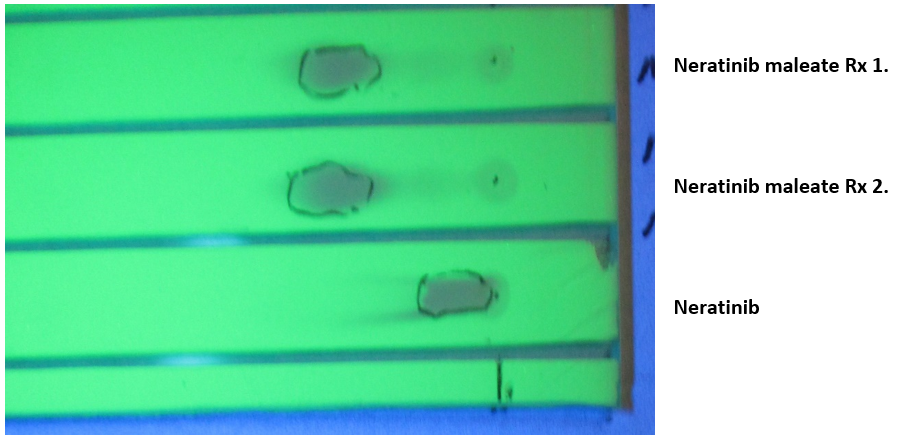
Figure 1: Thin layer chromatogram of Neratinib compared to two reaction preparations of the maleate salt. LK5DF plate observed under 254 nm UV light, 10ul spots of a 4 mg/ml in methanol/20mM ammonium formate, pH 5.1, 60/40, v/v, eluting phase: 55/45; EtOH/H2O
Table 1:Neratinib salt preparation
|
Salt |
Formula |
M.W. |
Added acid (mg) |
Final pH |
Precipitated salt (mg) |
Yield |
|
Maleate |
C34H33ClN6O7 |
673.1 |
212 |
4.5 |
895 |
73.8% |
|
Succinate (EtOH) |
C34H35ClN6O7 -H2O |
693.1 |
213 |
5.2 |
1122 |
92.4% |
|
Succinate (IPA) |
C34H35ClN6O7 -H2O |
693.1 |
213 |
5.2 |
1120 |
92.4% |
|
Glutarate |
C35H37ClN6O7-H2O |
707.2 |
237 |
4.5 |
1099 |
88.8% |
|
l-Malate |
C34H35ClN6O8-H2O |
709.1 |
241 |
4.5 |
638 |
63.8% |
Figure 2: High performance liquid chromatograms for Neratinib succinate log P determination. Top: injection of 10 µl of the octanol phase ten times diluted, Neratinib salt peak area = 14574686. Bottom: injection of 10 µl of the octanol saturated aqueous phase, Neratinib salt peak area = 29566. P = 145746860/29566 = 4929. log P = 3.69. Column Shim-Pac 15 cm, 5 µm, UV detection 265 nm. Mobile phase: methanol/20 mM ammonium formate buffer, pH 5.1, 60/40, v/v. Flow rate 1 ml/min. at 40â. Two standard molar, four point calibration curves were prepared using a commercial standard Neratinib maleate obtained from Excella GmbH marked INP-33A-1 and our own prepared succinate salt. The concentration range was 0-0.7 mM or 0-480 mg/L for the succinate and 0-470 mg/L for the maleate salt. The salts were dissolved in HPLC mobile phase (methanol/20 mM ammonium formate buffer, pH 5.0, 60/40, v/v). Two straight lines (r2 > 0.98) obtained were identical having exactly the same slopes with nil intercepts
Solubility Measurements
To weighed samples of each of the salts were added incremental amounts of the various solvents with stirring and sonication induced after each addition for a minimum of 10 minutes. Once the solution appeared clear, calculations of the solubility were made. During these studies there was a significant difference in the solubility studies between the ethanol crystallized succinate and the isopropanol crystallized succinate. See Table 2. Repeated preparations came to the same conditions. The possibilities of the two crystalline forms being polymorphs have been raised. We concluded that if one of the carboxyl groups was unassociated due to a binding of the ethanol to the strongest amine the difference might be explained especially given the large differences in solubility. FT-IR spectroscopy gave some indication that the latter is true but was too complex to be definitive. Regardless it is reproducible.
Octanol/water Distribution Coefficients
The shake flask method was used with presaturated 1-octanol and aqueous buffer of 20 mM potassium phosphate at pH 8.1. Neratinib salt (11 mgs) was introduced into a graduated 25 mL centrifuge tube. Octanol presaturated with 20 mM phosphate buffer (10 ml) was added and the tube was sonicated for 5 min. Next 5 mL of aqueous buffer was added and the entire mixture was shaken for 10 min. Visually from the yellow color, it appeared that most of the sample had gone into the upper organic layer. The salt concentration in the two phases was determined by HPLC as illustrated by Fig. 2 for a Neratinib succinate experiment. On injection of 10µL of the clear yellow octanol layer, an off scale peak was obtained. The yellow octanol phase was therefore diluted 1:10 with pure 1-octanol to determine the salt concentration. The log P was determined as the ratio of the salt concentration obtained in the buffered octanol phase over that in the buffer aqueous phase. The log P’s are given in Table 2.
Table 2:Neratinib salts solubility
|
Salt |
Stable solvent solubility mg/mL |
log P(av.) |
|
|
Ethanol |
60% aq. DMSO |
||
|
Maleate |
4.7 |
5.5 |
3.16 |
|
Succinate (EtOH) |
12.2 |
21 |
3.67 |
|
Succinate (IPA) |
5.4 |
NS* |
3.78 |
|
Glutarate |
9.2 |
11 |
3.47 |
|
l-Malate |
17.5 |
9.1 |
2.71 |
*NS - soluble only in 75% aqueous DMSO @ 3.7 mgs/ml. Concentration increases as DMSO concentration increases. In 100 % DMSO it is soluble at 7.7 mgs/ml. Must be dissolved in DMSO first and then diluted with water. Also note the maleate salt is the approved drug formulation.
Results and Discussion
Considering the good results obtained by Neratinib maleate in blocking HER2+ breast cancer metastases in the body but not sufficiently in the brain [2-6], different ways to administer the drug for brain metastases must be sought. The only preparation containing Neratinib, is administered orally in the form of the maleate salt with a very common diarrhea side effect due to its low oral adsorption rate. Since Neratinib maleate does not seem able to effectively cross the BBB in case of cranial HER2+ metastases, other Neratinib salts had to be tested. Three of the salts of organic diacids were compared to Neratinib maleate for solubility and hydrophobicity.
Diacid Neratinib Salts Solubility
Table 2 present the synthesized Neratinib salt solubility in ethanol, and aqueous 60% DMSO. The four newly synthesized salts have a higher solubility than the Neratinib maleate in the tested solvents. Neratinib succinate with its highest solubility in the 60% DMSO solution is promising for a galenic transdermal preparation both in crystal form from ethanol and isopropyl alcohol. As a dry sample the ethanol crystalized was not stable for long periods of time but had preferred solubility characteristics while the IPA crystal was stable indefinitely in dry state. Both appeared stable indefinitely in the aqueous DMSO. Diluting the water with absolute ethanol as a 20/20/60; H2O/EtOH/DMSO solution further increases the Neratinib succinate solubility to 44 mgs/ml. Since ethanol is also a good BBB transporter the combination would further enhance the transport of Neratinib salt. This solution is also quite stable. After three months of open exposure to ambient conditions the ethanol crystallized salt had the same solubility characteristics as the isopropyl alcohol crystallized salt. This gives further credence to the fact that it is an ethanol hydrogen bonding phenomena rather than a polymorph. In solution, however, they all retained their good solubility characteristics.
Diacid Neratinib salt octanol/water partition coefficient
Chromatograms of the upper octanol and lower aqueous phases obtained in a distribution coefficient, log P measurement, are shown in Figure 2. The isocratic condition with the selected 60/40 methanol/buffer mobile phase produces a Neratinib peak after about 7 min retention time. This delay would be sufficient to elute all polar components found in a biological sample so that the Neratinib concentration stays accurately determined. Table 2 lists the measured octanol/water distribution constant in the form of log P correlating to the salt lipophilicity. The listed values are the average values obtained after a number of experiments like the one illustrated by Fig. 2. As expected, when comparing the lipophilicity of a base to its salt, a number of salts have higher log P than the 3.16 value of Neratinib maleate. Again, Neratinib succinate with the highest log P seems to be the best candidate with a high enough lipophilicity that should allow effective BBB transmission.
Proposal for an efficient galenic transdermal Neratinib preparation
These results show that the active principle to treat HER2+ breast cancer brain metastases should be the ethanol crystallized Neratinib succinate dissolved in 20/20/60; H2O/EtOH/DMSO such that the next stage in this study would be to carry out an in vivo test of transdermal drug delivery. Methyl cellulose is an innocuous thickener that can be added to the solution to make it easy to handle and apply. The patient skin must be cleaned with a sterilizing swab just prior to application of the drug preparation. The person applying the preparation must be wearing butyl rubber gloves since other types of gloves will be dissolved by DMSO. The area must be free of supplemental oxygen as it has been reported that the DMSO can convert in the presence of high oxygen to dimethyl sulfone then to dimethyl sulfate a toxic oily liquid. The best protocol has to be established. Suitable sterilization method for DMSO applications can be found in reference.25 A classical protocol for transdermal application could be to apply 20 mL of the preparation dosed at 4 mg Neratinib succinate per mL (3.2 mg Neratinib base or 64 mg or 0.115 mmoles in 20 mL) from the base of the skull down the spinal cord to the base of the spine. This is one third of the usual oral dose. The application area should remain uncovered and undisturbed for about 15 min. Then, the skin can be wiped clean and immediately followed by a direct application of an Aloe vera preparation. Aloe vera gels were recommended for their anti-inflammatory and wound healing properties to avoid irritation or desquamation.26 The blood stream should be tested for Neratinib content after 15 minutes. Two main issues that will control the penetration of the drug, First is the concentration of the drug in the DMSO and second the percentage of the DMSO in water. The concentration of drug will depend on how much of a surface needs to be affected. As the DMSO concentration increases, the endothelial membrane will open wider. You want to keep this number as low as possible to reduce the effect on the skin surface that may require repeated applications.
Factors to consider to optimize outcome include the following:
- Percentage of DMSO.
- Concentration of the drug within is solubility range.
- Possibly buffering the DMSO. We noted that if we buffer the DMSO with succinic acid (pH 4) all of the Neratinib succinate went into the aqueous layer.
- Size of the area for the dermal application.
- The required contact time. While we suggest 15 minutes to begin with, the subsequent blood test should identify the need for greater or less contact time.
- The use of ethanol to further dilute the DMSO to enhance solubility also needs to be address.
Conclusion
In order to transfer the drug from the DMSO to the capillary blood system and remain stable some solubility at the physiological pH of 7.45 is required. The ethanol crystallized succinate is soluble at 1.875 mg/ml at pH 7.45 while the Neratinib maleate used in the partially successful clinical trials for brain penetration was ~0.4mg/ml. Ethanol crystalline Neratinib succinate has been shown to have the optimum lipophilicity and solubility for effective BBB transfer and as such to potentially provide effective treatment of HER2+ intracranial metastases. The proposed transdermal Neratinib succinate treatment should be significantly more efficient that the classical oral administration of Neratinib maleate with its low oral digestion. The work of Kolb, et al 27 has demonstrated that in man radioactive DMSO (S35) appeared in the blood five minutes after cutaneous application and in the bones after one hour. Rat studies demonstrated that two hours after skin application DMSO was detected in every organ. Since the oral route is by-passed, the associated common gut or intestinal problems will be avoided for the comfort and safety of the patient. Timing of the exposure may also be an important issue. Applying the transdermal treatment much closer to the targeted metastases will allow for the capillary system uptake, optimizing Neratinib efficiency. Keeping the dose at its minimum further reducing possible side effects. With more than 1.7 million new cases of breast cancer per year with 320,000 cases belonging to the HER2+ subtype, this cancer is the most common in women worldwide.28 If the proposed transdermal protocol could save only one woman, the authors would be most happy. In memory of Claudine Venne, whose unfortunate early passing inspired this work. Thanks also to Shimadzu for their generosity and help for the HPLC equipment and Drs Alain Berthod and Denise Wallworth for their technical inputs.
Conflict of Interest
The authors declare that there are no competing interests, and the work was self-funded in our own facility.
References
- Bachelot T, Romieu G, Campone M, et al., Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single group phase 2 study. Lancet Oncology. January 2013, Vol.14, Issue (1), pages 64-71.
- Ashraf M, Mainuddin M, Chimanlall G, et al. Coated tablet formulations and uses thereof, U.S. Patent 8,518,446 to Wyeth LLC, Madison, NJ. August 27, 2013.
- Freedman RA, Gelman RS, Wefel JS, et al., A Phase II Trial of Neratinib for Patients with Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases. J. Clin. Oncol. March 20, 2016, Volume 34, Issue 9, pages 945-952.
- Chan A, Neratinib Reduces HER 2+ Breast Cancer Recurrences in Phase III ExteNET Study. Lancet Oncology. March 2016, Volume 17, Issue 3 pages 367-377.
- Zhao Xiao-qin, et al., Neratinib Reverses ATP-Binding Cassette B1-Mediated Chemotherapeutic Drug Resistance In Vitro, In Vivo and Ex Vivo, Mol. Pharmacol., April 2012, Volume 82, Issue (1), pages 47-58. also www.ncbi.nih.gov.
- Ustaris F, Saura C, Di Palma J, et al. Effective Management and Prevention of Neratinib-Induced Diarrhea. Am. J. Hematol/Oncol. November 2015, Volume 11, Number 11, pages 13-22.
- Gaylord Chemical Company, Tuscaloosa, AL, Safety Data Sheet.
- Kurihara-Bergstrom T, Flynn GL, and Higuchi,WI. Physicochemical Study of Percutaneous Adsorption Enhancement by Dimethyl Sulfoxide: DMSO Mediation of Vidarabine (ara-A) Permeation of Hairless Mouse Skin, J. Investigative Dermatology, September 1987, Volume 89, Issue (3), pages.274-280.
- Capriotti K, Capriotti JA, Dimethyl Sulfoxide; History, Chemistry and Clinical Utility in Dermatology. J. Clin. Aesthet. Dermatol., September 2012, Volume 5, Issue (9) pages 24-26.
- Kulah A, Akar M, Baykut L. Dimethyl sulfoxide in the management of patients with brain swelling and increased intracranial pressure after severe closed head injury, Neurochirurgia, November 1990, Volume 33, Issue (6), pages 177-180.
- Karaca M, Bilgin UY, Akar M, et al. Dimethyl Sulfoxide Lowers ICP After Closed Head Trauma, Eur. J. Clin. Pharmacol., 1991Volume 40, Issue1, pages 113-114.
- McKim AS, Strub R, Dimethyl Sulfoxide USP, PhEur in Approved Pharmaceutical Products and Medical Devices, Pharm. Technol., May 2, 2008, Volume 32, Issue (5) pages 26-35.
- Marren K, Dimethyl sulfoxide: an effective penetration enhancer for topical administration of NSAIDs. Phys Sportsmed. Sep; 39 (3): 75-82, 2011.
- Baynes RE. and Hodgson E. Adsorption and Distribution of Toxicants. Chapter 6, A Textbook of Modern Toxicology, 3rd edition, Wiley and Sons, Inc., 2004.
- Bos JD, Marcus M, Meinardi HM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. December 24, 2001, Volume 9, Issue (3), pages 165-169.
- Quinhong Lu, et al., U.S.Patent 9139558 B2: Maleate salts of (E)-N-{4-[3-chloro-4-(2-pyridinylmethoxy)anilino]-3-cyano-7-ethoxy-6-quinolinyl}-4-(dimethyamino)-2-butenamide and crystalline forms thereof. Sept.22, 2015.
- Jacob SW, Herschler R. Pharmacology of DMSO, Department of Surgery, Oregon Health Science University, Portland, Oregon. www.dmso.org/articles/information/herschler.htm
- Jacob SW. Current Status of Dimethyl Sulfoxide (DMSO), Department of Surgery, Oregon Health Sciences University. 2002, consulted on Feb. 23, 2021.
- Pardridge WM. Blood-Brain Barrier: Bottleneck in Brian Drug Development, NeuroRx, January 2005, Volume 2, Issue (1), pages 3-14.
- Dash Pramod. Blood-Brain Barrier, Neuroscience Online, Chapter 11, Neuroscience.uth.tmc.edu/s4/chapter 11.html.
- Mikitsh JL, Chacko, Ann-Marie. Pathways for Small Molecule Delivery to the Central Nervous System Across the Blood-Brain Barrier, Perspectives in Medicinal Chemistry, June 2014, Volume 6, Issue (6) pages 11-24.
- Wong AD, Ye M, Levy AF, et al., The blood-brain barrier: an engineering perspective, Neuroengineering, 30 August 2013, Volume 6, Article 7, pages 1-22.
- Neuwelt EA, Barnett P, Barringer, et. Al. Neurosurgery, January 1983, Volume 12 Issue (1)., pages 29-34.
- Broadwell, et al., Science Jul.9, 1982, 217 (4555): pages 164-166.
- Caton, L.Jones A, Johnson A. Suitable Sterility Methods for Dimethyl Sulfoxide USP, PhEur, Pharmaceutical Technology September 1, 2020, Volume 2020 Supplement, Issue 4, pages 19-23.
- Surjushe A, Vasani R, Saple DG. Aloe Vera: A Short Review, Indian J. Dermatol., March 2008, Volume 53, Issue 4, pages 163-166.
- Kolb KH, Janiche G. Kramer M, et al. Adsorption, distribution and elimination of labeled DMSO in man and animals, 1967, Ann.N.Y.Acad Sci. 141:85095.
- World Cancer Research Fund International, Breast Cancer Statistics, https://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/breast-cancer-statistics%20 consulted on Feb. 23, 2021.
