Anesthetic Aspects of Cytoreductive Surgery and Hyperthermic Intrathoracic Chemotherapy (HITHOC) in Treatment of Pleural Malignancies - Experiences from IORS
Ana Cvetkovic MD PhD1,3*, Dejan Stojiljkovic MD Phd2,3*, Dijana Mircic MD PhD1*, Nada Santrac MD PhD2,3, Milan Zegarac MD PhD2,3, Andrej Jokic MD1, Lazar Glisic MD PhD4
1Department of Anesthesiology with Reanimatology and Intensive Care Unit, Surgical Oncology Clinic, Institute for Oncology and Radiology of Serbia, Belgrade, Serbia
2Department of Surgery, Surgical Oncology Clinic, institute for Oncology and Radiology of Serbia, Belgrade, Serbia
3Medical School, University of Belgrade, Belgrade, Serbia
4University Hospital for Obstetrics and Gynecology, Medical Faculty, Otto-von-Guericke University, Magdeburg, Germany
*All authors contributed equally as first authors
Abstract
Background: Cytoreductive surgery (CRS) with hyperthermic intrathoracic chemotherapy (HITHOC) is a procedure that includes surgical removal of all visible tumor implants and intrathoracic application of gradually heated cytostatic solution. Numerous changes in hemodynamic, respiratory, core body temperature and metabolic parameters are possible during this complex procedure. The aim of this retrospective study was to analyze pathophysiological changes which occur during CRS + HITHOC procedure, and to suggest efficient strategies for perioperative patient care that might reduce complication rate.
Methods: The study included 7 patients who underwent CRS + HITHOC in our cancer center. Enhanced Recovery After Surgery (ERAS) guideline for Thoracic Surgery was applied to all patients. Data on intraoperative hemodynamics (mean arterial pressure, stroke volume, heart rate, cardiac output) and temperature variations were collected from medical records and analyzed in three timelines: during the CRS phase, at the beginning, and the end of cytostatic perfusion. Occurrence of respiratory, renal, and cardiac complications was monitored.
Results: All patients were respiratory stable during one-lung ventilation, with adequate gas exchange. Hemodynamic stability was compromised at the beginning of cytostatic perfusion, with significant decrease of mean arterial pressure and stroke volume. Two patients required vasopressor support. Average core body temperature was satisfactory in all patients. Coagulation disorders and acute renal failure were not recorded in postoperative period. One patient developed atrial fibrillation which was successfully pharmacologically restored.
Conclusion: Our results indicate that goal directed fluid management following ERAS protocol, with maintaining hemodynamic stability and normothermia, could prevent perioperative complications during HITHOC procedure.
Introduction
The combination of cytoreductive surgery (CRS) and hyperthermic intrathoracic chemotherapy (HITHOC) is used in treatment of primary and secondary pleural malignancies in well-selected patients1. CRS implies removal of macroscopically visible pleural lesions, while HITHOC aims to eliminate potential microscopic disease, thus improving local disease control and survival rates2,3. The application of hyperthermic solution of cytostatic agent in the thoracic cavity improves permeability and local cytotoxic effect of the drug, while systemic toxicity is reduced1,4.
CRS + HITHOC is a challenging procedure, not only for a surgical team, but also for anesthesiologists. During perioperative course, numerous variations in hemodynamics (hypotension, tachycardia, cardiac arrhythmias), respiratory variables, temperature, and metabolic and coagulation parameters are possible5. Adverse events in perioperative period are a result of hypothermia and redistribution of fluids during cytoreductive surgery, while in the second phase - during perfusion - they are a result of rapid increase of intrathoracic pressure, regional and systemic hyperthermia, as well as toxicity of cytostatic agents6.
Consequently, main anaesthetic goals are monitoring and maintaining hemodynamic stability of a patient with targeted fluid management according to Enhanced Recovery after Surgery (ERAS) protocol as well as prevention of acidosis by maintaining normothermia and normal gas exchange7.
The aim of this retrospective study was to analyze pathophysiological changes which occur during CRS + HITHOC procedure, as well as to suggest efficient strategies for perioperative patient care that might reduce complication rate.
Materials and Methods
This retrospective study was carried out at the Surgical Oncology Clinic of the Institute for Oncology and Radiology of Serbia (IORS). It included patients who underwent CRS + HITHOC at our cancer center in the period from January 1st, 2018 until August 1st, 2021.
Data on patients and treatment were collected from medical records with approval from the IORS Ethical Committee and analyzed using Microsoft Excel. Parameters of specific interest were intraoperative hemodynamics: mean arterial pressure (MAP), stroke volume (SV), cardiac index (CI), heart rate (HR), cardiac output (CO); metabolic parameters (lactates); temperature variations; respiratory, renal and cardiac complications. These were analyzed in three timelines: during the CRS phase, at the beginning, and the end of cytostatic perfusion.
CRS and HITHOC procedure
As a standard protocol at IORS, patients are selected for the CRS + HITHOC procedure by the multidisciplinary team. Informed consent is obtained for all patients before the treatment. CRS is performed by removing all macroscopically visible tumor implants and/or tumor mass through posterolateral thoracotomy in the sixth intercostal space or medial sternotomy. Following, chest tubes are placed into the thoracic cavity of the unventilated lung, the chest is closed and perfusion with priming solution (1000 ml of 5%/glucose) is started at an initial temperature of 38°C. The solution is gradually heated to 41°C when Cisplatine (Sinplatin®, Actavis Italy S.P.A.) is added (100 mg/m2) and cytostatic perfusion is continued for 45 minutes. Normothermia is maintained with cold solutions and an air blanket.
Specifics of anesthesia management for HITHOC procedure
Preoperative evaluation of cardiopulmonary reserve is performed in all patients with clinical examination, echocardiogram, six-minute walk test, and estimation of dynamic and functional reserve of the respiratory system (spirometry and blood gas analysis).
Based on IORS protocols for patients receiving HITHOC, on the day of surgery, all patients are given double antiemetic therapy and antibiotic prophylaxis. The thoracic epidural catheter is placed at level Th 8-9.
Patients are placed in a lateral position onto a heating/cooling blanket (Mistral-air Plus). General anesthesia is induced with Propofol (Propofol Lipuro, Braun Melsungen AG), Fentanil (Fentanyl, GlaxoSmithKline Manufacturing S.P.A.) and Rocuronium (Esmeron®, N.V. Organon). Afterward, it is maintained with Sevoflurane (Sevorane®, Abbvie S.R.L.), which is titrated to maintain the minimal alveolar concentration of 0,8 and bispectral index values (BIS) between 40-60.
After a double-lumen tube is placed, protective one-lung ventilation is started with 5 ml/kg tidal volume, limited plateau pressure, and positive end-expiratory pressure of 5 cmH20. The values of expiratory end-tidal CO2, pressure in the respiratory tract, and urine output are constantly monitored.
A nasogastric tube, central venous line, arterial line, esophageal temperature probe, and urinary catheter are routinely placed on all patients. The Esophageal Doppler probe (CardioQ) is used to monitor changes in stroke volume and guide fluid management according to the ERAS hemodynamic algorithm7.
Hemodynamic parameters MAP, SV, and CI are standardly measured by esophageal Doppler probe, and initial input values are recorded as basal. The first “fluid challenge” is done with a 200 ml bolus of crystalloid solution. Depending on the response, the bolus of crystalloid solution is repeated if the stroke volume is increased by 10%. If MAP Ë 70 mmHg, CIË 2,5 l/min/m² and SV do not change more than 10% after fluid bolus, norepinephrine in continuous infusion is used (Figure 1). If the cardiac index remains below 2.5 l/min/m2 and SV remains the same after fluid boluses positive inotropic drug is used8. Hemodynamic parameters are then recorded in three timelines: during the CRS phase, at the beginning, and the end of cytostatic perfusion.
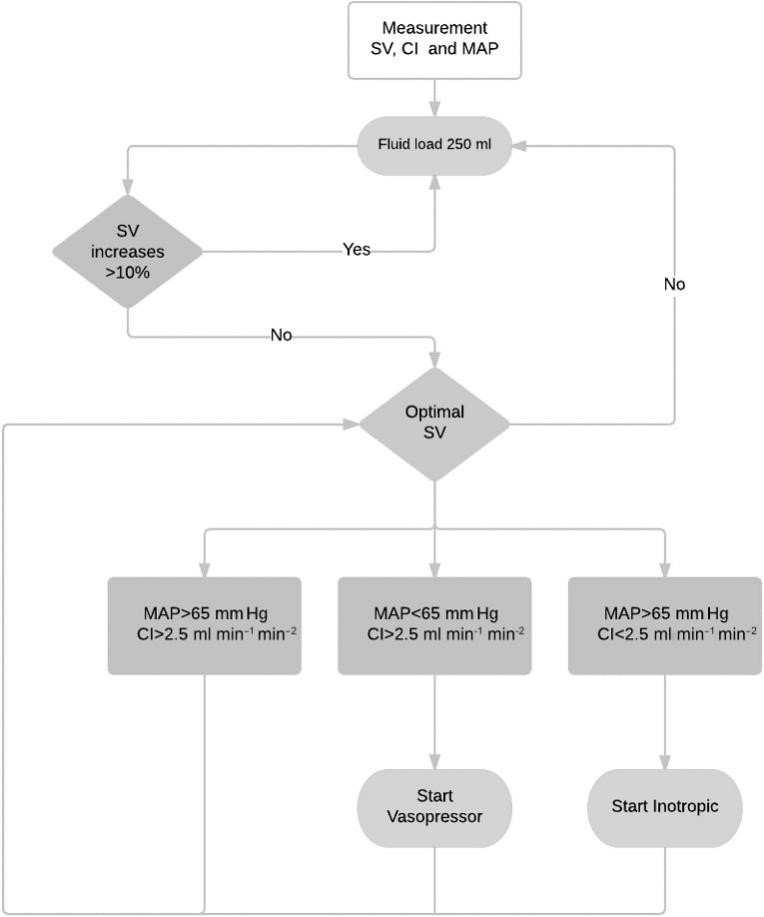
Figure 1. ERAS algorithm for guiding intraoperative fluid management during HITHOC (addapted from: Calvo-Vecino JM, Ripollés-Melchor J, Mythen MG, Casans-Francés R, Balik A, Artacho JP, Martínez-Hurtado E, Serrano Romero A, Fernández Pérez C, Asuero de Lis S; FEDORA Trial Investigators Group. Effect of goal-directed haemodynamic therapy on postoperative complications in low-moderate risk surgical patients: a multicentre randomised controlled trial (FEDORA trial). Br J Anaesth. 2018 Apr;120(4):734-744. doi: 10.1016/j.bja.2017.12.018.)
Legend: CO, cardiac output; SV, stroke volume; CI, cardiac index; MAP, mean arterial pressure.
After the HITHOC procedure, patients are routinely transferred to the intensive care unit where the occurrence of respiratory, renal, and cardiac complications is monitored. Renal complications are assessed and ranked according to RIFLE (Risk, Injury, Failure, Loss, and End-stage renal disease) criteria which are based on changes in serum creatinine and level of urinary excretion9.
Results
Out of total of 7 patients that underwent CRS + HITHOC procedure, complete data were available only for six, thus they were included in this study. Mean age in this series of patients was 40.7 ± 19.14 (range: 16-62) years, with equal gender distribution (Table 1). Four patients had primary or metastatic pleural tumors and two were diagnosed with thymoma.
Table 1: Patients’ characteristics
|
Parameters |
N (%) or mean ± SD (range) |
|
Age |
40.7±19.1 (16-62) |
|
Gender Female Male |
3 (50%) 3 (50%) |
|
Weight |
68.5 ± 9.8 (50-114) (kg) |
|
Height |
172.2 ± 5.1 (153-183) (cm) |
|
BMI |
23.2 ± 3.1 (14.9-36) (kg/m²) |
|
ASA I II III IV V |
0 4 (66.7%) 2 (33.4%) 0 0 |
N - number of patients, SD – standard deviation, BMI - body mass index, ASA - American Society of Anesthesiologists
During one-lung ventilation, oxygenation and gas exchange were satisfactory in all patients. None of them developed respiratory complications intraoperatively. Hemodynamic parameters were maintained in reference range until the beginning of cytostatic insertion (“priming”) into thoracic cavity. After “priming” and due to increase in intrathoracic pressure, there was a significant decrease of MAP and SV in all patients, while hypotension was followed by tachycardia in two patients (33%). Regarding those two patients, fluid bolus did not give satisfactory response, so we continued with vasopressor. Norepinephrine was used 0.05 - 0.1 µg/kg/min during whole operation, after which we achieved hemodynamic stability in one, while the other one patient was moved to intensive care unit until hemodynamic stabilization. (Table 2)
Table 2: Intraoperative hemodynamic parameters during cytoreductive surgery and hyperthermic intrathoracic chemotherapy
|
|
HITHOC procedure phase |
||
|
Parameters |
T1 (AVG±SD) |
T2 (AVG±SD) |
T3 (AVG±SD) |
|
MAP |
73.24±5.55 |
73.83±9.26 |
76.26±5.66 |
|
HR |
78.17±12.21 |
87.00±11.38 |
84.35±9.47 |
|
CO |
5.66±0.71 |
4.76±1.07 |
5.85±0.64 |
|
SV |
73.83±13.33 |
54.19±11.78 |
71.5±5.61 |
|
CI |
3.23±0.75 |
2.76±0.90 |
3.44±0.59 |
|
Lac |
1.4 (1.3-1.6) |
2.55 (2.01-3.6) |
2.13 (1.8-3.0) |
|
BT |
35.88±0.36 |
38.12±0.39 |
36.90±0.56 |
HITHOC - hyperthermic intrathoracic chemotherapy, T1 - cytoreduction, T2 - beginning of cytostatic perfusion, T3 - end of cytostatic perfusion, MAP - middle arterial pressure, HR - heart rate, CO – cardiac output, SV - stroke volume, CI - cardiac index, Lac – lactates, BT - body temperature measured by esophageal probe, AVG – average, SD – standard deviation.
Average surgery duration was 312.50 ± 33.43 min, while average anesthesia lasted for 369.17 ± 49.03min.Average duration of each phase is shown in table 3. Average crystalloid solutions volume was 2000 ± 632.46 ml, while colloid solutions were not used in all patients. One patient received intraoperative blood transfusion due to increased intraoperative bleeding. Intraoperative fluid management is shown in Table 4.
Table 3: Average duration of HITHOC procedure phases
|
Phase 1 citoreduction |
Phase 2 beginning of cytostatic perfusion |
T3 - end of cytostatic perfusion to ICU transfer |
|
230 min |
45 min |
77 min |
Table 4: Intraoperative fluid management during cytoreductive surgery and hyperthermic intrathoracic chemotherapy
|
Parameter |
N |
Mean ± SD / range |
|
Crystalloids |
6 |
2000.00 ± 632.46 ml |
|
Colloids |
3 |
116.67 ± 129.10 ml |
|
FFP |
0 |
Not applicable |
|
RBC transfusion |
2 |
Not applicable |
|
Vasopressors* |
2 |
0.05 – 0.1 µg/kg/min |
|
Surgery duration |
6 |
312.50 ± 33.43 minutes |
|
Anesthesia duration |
6 |
369.17 ± 49.03 minutes |
N – number of patients, SD - standard deviation, FFP – fresh frozen plasma; RBC – red blood cells; * noradrenaline
Average core body temperature before beginning of perfusion by heated cytostatic solution was 35.5 ± 0.6°C. Soon after beginning of HITHOC, there was continuous increase of temperature in all patients despite active cooling with air blanket and cold crystalloid solutions. Average temperature was 38.1 ± 0.4°C (Figure 2).
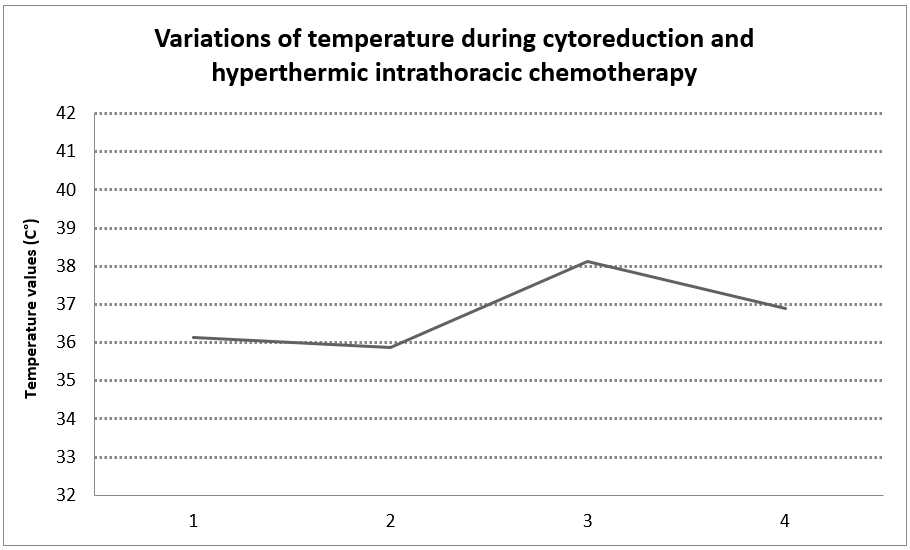
Figure 1: Variations of temperature during cytoreduction and hyperthermic intrathoracic chemotherapy.
HITHOC - hyperthermic intrathoracic chemotherapy, interval 1-2: cytoreduction; interval 2-3: cytostatic perfusion, interval 3-4: end of HITHOC
Five patients were extubated in the operating room, while one patient was on prolonged mechanical ventilation in intensive care unit and extubated on the same day.
There were no recorded coagulation disorders and acute renal failure in the postoperative period. One patient developed atrial fibrillation which was successfully pharmacologically restored. Average hospitalization period in the intensive care unit was 6.50 ± 0.50 days, while the length of hospital stay was 13.17 ± 4.45 days.
Discussion
Indications for CRS + HITHOC have so far been reserved for malignant mesothelioma and advanced thymoma. However, the indications for this complex procedure are expanding and positive effects of HITHOC in other secondary pleural malignancies, such as metastatic ovarian cancer, peritoneal pseudomyxoma and sarcoma were also reported10-12. Experiences with HITHOC are mostly published as case reports and limited series of patients, showing variations in the choice of cytotoxic drug, it’s concentration, volume and temperature of the perfusion system and duration of the drug circulation1.
There are insufficient publications about anesthesiology aspects of this method. Thoracic anesthesia is already very challenging, while CRS + HITHOC further increases the risk of perioperative complications. The main anesthetic concerns during this procedure are hemodynamic stability with adequate organ perfusion, maintaining of normothermia, adequate gas exchange and reduction of the cytostatic-associated risks5.
During CRS phase, hypovolemia can occur due to bleeding during decortication. We had one patient that required intraoperative blood transfusion, but without postoperative coagulation disorders.
Pathophysiological changes are the most notable in the second HITHOC phase, during cytostatic perfusion. The biggest hemodynamic and respiratory disbalance is caused by increase of intrathoracic pressure which further increases respiratory pressure, leads to mediastinal shift and reduction of functional residual lung capacity. Preload is decreased due to compression of the superior and inferior vena cava, as well as direct compression of circulating fluid on the heart. Coronary perfusion pressure is also decreased, increasing myocardial oxygen demand5.
Optimal fluid management in thoracic surgery and its effect on postoperative pulmonary complications still represent one of the major debates among experts. It has been clearly shown that liberal fluid management leads to acute lung injury and pulmonary edema, not only in pneumonectomy, but also in minor lung resections13. Restrictive fluid management, on the other hand, is very common in thoracic surgery, with the aim to reduce hydrostatic pressure in pulmonary capillaries. Evans et al.14 concluded that intraoperative infusion rate of 1-2 ml/kg/h is optimal for perioperative maintenance of euvolemia, while the risk of postoperative pulmonary edema and acute respiratory distress syndrome can be reduced, but there is the risk of hypovolemia and tissue hypoperfusion, as well as acute renal failure in postoperative period. However, Budacan et al.15 have shown that euvolemic strategy is the best method in perioperative fluid therapy, which is in accordance with the current recommendations by European Society of Thoracic Surgeons7. Considering the fact that all our patients underwent only minor resections of lung parenchyma, while, one the other hand, cytostatic agents can lead to kidney damage, our approach was to use of goal directed fluid therapy in order to optimize oxygenation and tissue perfusion, while maintaining euvolemia.
The choice of vasoactive drug depends on hemodynamic findings. Some authors use inotropes more frequently than vasopressors for maintaining hemodynamic stability in order to avoid volume overload16. According to our protocol that was previously described in detail in the Methods section, we have adjusted fluid therapy and norepinephrine use to the response to initial crystalloid solution bolus assessed through monitoring MAP, CI and SV. To our experience, euvolemic strategy is adequate choice for CRS + HITHOC procedure considering that we did not experience complications that follow volume overload.
Another important goal during CRS + HITHOC procedure is to maintain normothermia. In the first phase, during CRS, the patients are at risk of developing hypothermia. Afferent and efferent control of thermoregulation is poorer when general and regional anesthesia are combined since there is redistribution of heat from central towards peripheral compartments17. In addition to impaired thermoregulatory mechanisms, there is a significant heat loss as a result of radiation and convection during this long procedure. In order to prevent the central temperature drop, prior to anesthesia induction we start active warming of the patient with heating/cooling blankets, which is stopped immediately before the introduction of the heated cytostatic agent.
In the second phase, during perfusion of cytostatic that is heated to 41°C, patients are at risk of developing hyperthermia. Core body temperature can be increased even up to 40.5°C, suggesting that active cooling is mandatory18. Inadequate central temperature control can cause a range of complications in intraoperative, as well as postoperative period. Hyperthermia increases metabolic oxygen demand, as well as carbon dioxide production, which can deteriorate hypoxemia and cause respiratory acidosis due to hypercapnia19. The patients with associated cardiovascular diseases and reduced cardiovascular reserve are at the greatest risk. When core body temperature is above 39°C, the risk of developing malignant arrhythmias, acute myocardial infarction and neurological complications is significantly higher20. The average core body temperature in our patients was 38.6°C while the temperature of the intrathoracic cytostatic solution remained at 41°C. There was no evidence of any complications that can be related to hyperthermia. Atrial fibrillation occurred in one patient on the third postoperative day, which was successfully pharmacologically restored. Given that our patients didn’t have any significant lung parenchyma resection, which can be associated with higher incidence of atrial fibrillation we can only speculate that this complication occurred as the consequence of toxic effect of heated cytostatic, as previously shown in literature21,22.
Lung protective ventilation with low tidal volume and addition of positive end expiratory pressure is essential to maintain adequate gas exchange6,11,13. This way we ensured adequate oxygenation and prevented hypoxemia in our patients.
Previous studies have shown acceptable perioperative complication rates related to CRS + HITHOC. Acute renal failure (ARF) is considered to be clinically the most important one. It occurs as the result of cytostatic absorption into the systemic circulation, which leads to acute tubular necrosis. Prevention of ARF includes adequate hydration and, if necessary, application of diuretics23. The choice of hemiotherapeutic is imperative as some, such as Cisplatin, are more nephrotoxic than others. This has led to developing of nephroprotective regiments with different hemiotherapeutics and protective drugs like amifostine which when combined with forced diuresis shows a reduction in renal complications. While Cisplatin can be used with amifostine to reduce complications, it can also be used as a single agent below its maximum tolerable dosage of 225mg/m2 24 and still offer satisfactory results. We did not observe any complications of this kind in the perioperative period. Along with ARF, pulmonary embolism, atrial fibrillation, interstitial pneumonitis, pneumonia, postoperative pleural effusion and pleural empyema are outlined in literature as possible complications in the second HITHOC phase and postoperative period16. Out of these, we have recorded atrial fibrillation in one patient which was successfully pharmacologically managed.
Considering the patients in the study were not eligible for other therapeutic modalities, the CRS + HITHOC represents innovative approach to pleural malignity’s treatment. It is important to understand pathophysiological alterations to avoid or minimize potential complications and to improve perioperative patient care. Liberal or restrictive fluid management can easily lead to complications described in this specific procedure. To our opinion, euvolemic strategy in fluid management, along with temperature balance, represent cornerstone of CRS + HITHOC.
Author Contributions:
Ana Cvetkovic MD PhD, Dejan Stojiljkovic MD PhD, and Dijana Mircic MD PhD contributed equally to this work.
A. Cvetkovic: Conceptualization, Methodology, Formal Analysis, Investigation, Writing – Original Draft, Review & Editing.
D. Stojiljkovic: Conceptualization, Methodology, Data Curation, Validation, Writing – Original Draft, Review & Editing.
D. Mircic: Conceptualization, Investigation, Visualization, Writing – Original Draft, Review & Editing.
All authors participated in the critical review and final approval of the manuscript. They have given their final approval for the submission and agreed to be accountable for all aspects of the work, ensuring its accuracy and integrity.
Prior Presentations: Not applicable.
Conflicts of interest: No potential conflict of interest relevant to this article was reported.
Funding sources: none.
IRB number: Not applicable.
Clinical trial registration number: Not applicable.
References
- StojiljkoviÄ D, SantraÄ N, MirÄiÄ D, et al. Cytoreductive surgery and hyperthermic intrathoracic chemotherapy (HITHOC) in the treatment of primary and metastatic pleural malignancies - is extension of indications possible? J BUON, 2021; 26(6): 2658-2663.
- Sugarbaker PH, Chang D, Stuart OA. Hyperthermic intraoperative thoracoabdominal chemotherapy. Gastroenterol Res Pract. 2012; 2012: 623417. doi: 10.1155/2012/623417. Epub 2012 May 10. PMID: 22654899; PMCID: PMC3357938.
- Yellin A, Simansky DA, Paley M, et al. Hyperthermic pleural perfusion with cisplatin: early clinical experience. Cancer. 2001 Oct 15; 92(8): 2197-203. doi: 10.1002/1097-0142(20011015)92:8<2197::aid-cncr1563>3.0.co; 2-f. PMID: 11596038.
- Ried M, Potzger T, Braune N, et al. Cytoreductive surgery and hyperthermic intrathoracic chemotherapy perfusion for malignant pleural tumours: perioperative management and clinical experience. Eur J Cardiothorac Surg. 2013 Apr; 43(4): 801-7. doi: 10.1093/ejcts/ezs418. Epub 2012 Aug 10. PMID: 22885228.
- Kerscher C, Ried M, Hofmann HS, et al. Anaesthetic management of cytoreductive surgery followed by hyperthermic intrathoracic chemotherapy perfusion. J Cardiothorac Surg. 2014 Jul 25; 9: 125. doi: 10.1186/1749-8090-9-125. PMID: 25059994; PMCID: PMC4123496.
- Gómez Tarradas JM, Pujol Fontrodona G, López-Baamonde M, et al. Perioperative anesthetic management of patients with malignant pleural mesothelioma undergoing cytoreductive surgery and intraoperative chemotherapy. Rev Esp Anestesiol Reanim (Engl Ed). 2020 Jan; 67(1): 15-19. English, Spanish. doi: 10.1016/j.redar.2019.03.003. Epub 2019 Jul 26. PMID: 31353039.
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. 2019 Jan 1; 55(1): 91-115. doi: 10.1093/ejcts/ezy301. PMID: 30304509.
- Feldheiser A, Conroy P, Bonomo T, et al. Anaesthesia Working Group of the Enhanced Recovery After Surgery (ERAS®) Society; Enhanced Recovery After Surgery Society. Development and feasibility study of an algorithm for intraoperative goaldirected haemodynamic management in noncardiac surgery. J Int Med Res. 2012; 40(4): 1227-41. doi: 10.1177/147323001204000402. PMID: 22971475.
- Bellomo R, Ronco C, Kellum JA, et al. Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004 Aug; 8(4): R204-12. doi: 10.1186/cc2872. Epub 2004 May 24. PMID: 15312219; PMCID: PMC522841.
- Dang A, Mansfield P, Ilsin B, et al. Intraoperative hyperthermic intrathoracic chemotherapy for pleural extension of pseudomyxoma peritonei. J Cardiothorac Vasc Anesth. 2007 Apr; 21(2): 265-8. doi: 10.1053/j.jvca.2006.04.022. Epub 2006 Aug 23. PMID: 17418746.
- Farivar AS, Louie BE, Aye RW, et al. Extrapleural pneumonectomy for primary pleural mullerian tumor in a young woman. Ann Thorac Surg. 2012 Jan;93(1): e1-2. doi: 10.1016/j.athoracsur.2011.06.096. PMID: 22186481.
- Stojiljkovic D, Nikolic S, Cvetkovic A, et al. Hyperthermic intrathoracic chemotherapy (HITHOC) in ovarian carcinoma - a propos of a case. J BUON. 2018 Dec; 23(7): 153-155. PMID: 30722125.
- Assaad S, Popescu W, Perrino A. Fluid management in thoracic surgery. Curr Opin Anaesthesiol. 2013 Feb; 26(1): 31-9. doi: 10.1097/ACO.0b013e32835c5cf5. PMID: 23262471.
- Evans RG, Naidu B. Does a conservative fluid management strategy in the perioperative management of lung resection patients reduce the risk of acute lung injury? Interact Cardiovasc Thorac Surg. 2012 Sep; 15(3): 498-504. doi: 10.1093/icvts/ivs175. Epub 2012 May 22. PMID: 22617510; PMCID: PMC3422923.
- Budacan AM, Naidu B. Fluid management in the thoracic surgical patient: where is the balance? J Thorac Dis. 2019 Jun; 11(6): 2205-2207. doi: 10.21037/jtd.2019.05.75. PMID: 31372253; PMCID: PMC6626784.
- Kaufmann KB, Stein L, Bogatyreva L, et al. Oesophageal Doppler guided goal-directed haemodynamic therapy in thoracic surgery - a single centre randomized parallel-arm trial. Br J Anaesth. 2017 Jun 1; 118(6): 852-861. doi: 10.1093/bja/aew447. PMID: 28575331.
- Sessler DI. Perioperative heat balance. Anesthesiology. 2000 Feb; 92(2): 578-96. doi: 10.1097/00000542-200002000-00042. PMID: 10691247.
- Gupta N, Kumar V, Garg R, et al. Anesthetic implications in hyperthermic intraperitoneal chemotherapy. J Anaesthesiol Clin Pharmacol. 2019 Jan-Mar; 35(1): 3-11. doi: 10.4103/joacp.JOACP_93_18. PMID: 31057232; PMCID: PMC6495627.
- Suzuki S, Hotchkiss JR, Takahashi T, et al. Effect of core body temperature on ventilator-induced lung injury. Crit Care Med. 2004 Jan; 32(1): 144-9. doi: 10.1097/01.CCM.0000098857.14923.44. PMID: 14707573.
- Ramegowda JK, Salam MA, Nayak V, et al. Anaesthetic management of extra-pleural pneumonectomy and hyperthermic intrathoracic chemotherapy procedure. Indian J Anaesth. 2015 Dec; 59(12): 807-10. doi: 10.4103/0019-5049.171574. PMID: 26903675; PMCID: PMC4743305.
- Barash, Paul G. Clinical Anesthesia. 8th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2017.
- de Bree E, van Ruth S, Rutgers EJ, et al. Intraoperative hyperthermic intrathoracic perfusion chemotherapy for pleural metastases of thymic neoplasms. Eur J Surg Oncol. 2002 Sep; 28(6): 685-6. doi: 10.1053/ejso.2002.1323. PMID: 12374104.
- Markowiak T, Kerner N, Neu R, et al. Adequate nephroprotection reduces renal complications after hyperthermic intrathoracic chemotherapy. J Surg Oncol. 2019 Dec; 120(7): 1220-1226. doi: 10.1002/jso.25726. Epub 2019 Oct 10. PMID: 31602673.
