PD-L1 IHC 22C3 pharmDx Demonstrates Precision and Reproducibility in Triple-negative Breast Cancer
Brittany M Watts*, Monika D Vilardo, Christopher J La Placa, Judith Frederick, Siena Tabuena-Frolli, Malinka J Jansson, Kristopher J Kersch, Karina Kulangara, Kelly Martyniuk, Epiphani C Simmons, Stephanie A Hund
Agilent Technologies, Inc., 6392 Via Real, Carpinteria, California, USA
Abstract
In the United States, the PD-L1 IHC 22C3 pharmDx indications for use have expanded to include the assay’s use as an aid in identifying triple-negative breast cancer (TNBC) patients for treatment with pembrolizumab (KEYTRUDA®). Analytical validation for TNBC was conducted by Agilent Technologies in support of the device performance and FDA-approval. Analytical validation studies included device precision (inter-instrument, inter-operator, inter-day, inter-lot, and intra-run) and external reproducibility (inter- and intra-site, inter- and intra-observer) using the Combined Positive Score (CPS) ≥ 10 cutoff. All precision and external reproducibility studies achieved confidence interval lower bound values of greater than 85% for positive, negative, and overall percent agreement. These analytical validation studies demonstrate that PD-L1 IHC 22C3 pharmDx is precise and reproducible in evaluating PD-L1 protein expression at a CPS ≥ 10 cutoff when used for testing TNBC tissues.
Introduction
Triple-negative breast cancer (TNBC) lacks the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor type 2 (HER2), which consequently restricts the application of many suitable cancer therapies and treatments common to most breast cancer types1. In the absence of the major therapeutic targets ER, PR, and HER2, patients with TNBC are threatened with reduced survival rates compared to patients with breast cancers of other molecular subtypes2,3. TNBC has demonstrated an even lower survival rate in patients with late-stage cancer when compared with breast cancers exhibiting other molecular subtypes3. Furthermore, evidence of resistance to standard cytotoxic chemotherapy in TNBC patients limits the efficacy of current treatment, thus prioritizing the need to implement more specialized therapeutics targeting TNBC4. Elevated persistence in seeking alternative treatments for targeting TNBC may play an important role in increasing overall survival in patients diagnosed with this disease. Programmed cell death ligand 1 (PD-L1) is a protein and ligand of programmed cell death 1 (PD-1) and is expressed on numerous cell types including immune and tumor cells with expression observed across various cancer indications such as TNBC5-7. The immune-suppressive PD-1:PD-L1 signaling pathway is frequently exploited by tumor cells to increase proliferation, and thus, can be utilized as a target for anti-tumoral therapies7-10. Antibody targeting of the PD-1:PD-L1 signaling pathway could be beneficial for breast cancer patients with positive PD-L1 expression in invasive tumor cells and tumor-infiltrating lymphocytesparticularly those patients with TNBC8-11.
PD-L1 IHC 22C3 pharmDx (Agilent Technologies, Inc., Santa Clara, CA, USA) is a companion diagnostic (CDx) immunohistochemistry (IHC) assay using monoclonal mouse anti-PD-L1, Clone 22C3 intended for use in detecting PD-L1 protein expression in multiple cancer indications [in the United States, these include formalin-fixed, paraffin-embedded (FFPE) non-small cell lung cancer (NSCLC), esophageal squamous cell carcinoma (ESCC), cervical cancer, and head and neck squamous cell carcinoma (HNSCC) tissues] and has also been analytically validated using FFPE human TNBC tissue12-15. Using PD-L1 IHC 22C3 pharmDx, TNBC PD-L1 protein expression is determined by the Combined Positive Score (CPS) algorithm, which is the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by one-hundred13, [i]. CPS was first introduced for determination of PD-L1 expression in gastric or gastroesophageal junction (GEJ) adenocarcinoma using PD-L1 IHC 22C3 pharmDx[ii] and has since been employed for numerous indications12-15. Additionally, PD-L1 IHC 22C3 pharmDx has been implemented for determining PD-L1 expression in a Phase 3 clinical trial (KEYNOTE-355; ClinicalTrials.gov, NCT02819518) investigating pembrolizumab (KEYTRUDA®; Merck & Co., Inc., Rahway, NJ, USA) in combination with chemotherapy for patients with locally recurrent unresectable or metastatic TNBC, who had not been previously treated with chemotherapy in the metastatic setting16-19. From the overall study population, 38% of TNBC patients had tumor PD-L1 expression of CPS ≥ 1016-19. Importantly, KEYNOTE-355 demonstrated significant improvement in progression-free survival (PFS), thus decreasing the risk of disease progression or death by 35%16-19.
To demonstrate device performance, PD-L1 IHC 22C3 pharmDx undergoes rigorous testing prior to approval by the United States Food and Drug Administration (FDA). Analytical validation (referred to as validation) of the device using a new cancer type is therefore a prerequisite for FDA approval of that indication[iii]. TNBC validation was completed in two parts: internal and external validation (referred to as external reproducibility). All internal validation studies, including precision and repeatability, were performed at Agilent Technologies, Inc. (Carpinteria, California, USA) and were conducted using deidentified archival tumor specimens20. External validation studies, including assay reproducibility and observer reproducibility were performed at three College of American Pathologists (CAP) accredited and Clinical Laboratory Improvements Amendments (CLIA) licensed laboratories using archival tumor specimens20. The study results for validation of PD-L1 IHC 22C3 pharmDx using TNBC tissue represent device performance and support its intended use.
Materials and Methods
Tissue specimen preparation and selection
Sections from deidentified human TNBC FFPE tissue blocks were cut at 4 µm thickness, mounted on positively charged glass slides, oven-dried at 58 ± 2 °C for approximately 1 hour, and stored in the dark at 2-8 °C until staining (within 7.5 months of microtomy) with PD-L1 IHC 22C3 pharmDx on Autostainer Link 48 (Agilent Technologies)13.
Triple-negative status (ER-/PR-/HER2-) of human FFPE breast cancer specimens was prospectively confirmed by IHC staining for ER, PR, and HER2 expression prior to study inclusion. Using the Autostainer Link 48, expression of ER/PR was confirmed with the Dako ER/PR pharmDx Kit (Agilent Technologies) and HER2 was confirmed with HercepTest (Agilent Technologies)21, 22. Scoring of ER, PR, and HER2 staining was performed by certified pathologists.
Institutional Review Board (IRB) approval was obtained prior to conducting all studies and tissues were commercially obtained from tissue suppliers.
PD-L1 IHC staining procedure
A 3-in-1 procedure encompassing deparaffinization, rehydration, and target retrieval was performed using EnVision FLEX Target Retrieval Solution, Low pH (Agilent Technologies) to pre-treat all specimens in the PT Link module (Agilent Technologies). Autostainer Link 48, an automated IHC testing platform with a validated PD-L1 IHC 22C3 pharmDx (Code SK006) staining protocol, was used to perform all IHC testing of TNBC FFPE specimens13. The specimens were counterstained with Hematoxylin (Link) (Code K8008) (Agilent Technologies) and coverslipped using a non-aqueous, permanent mounting medium. All reagents and instrumentation were provided by Agilent Technologies.
Scoring of IHC tissue specimens
All specimens included in these studies were examined by certified pathologists or observers using a light microscope. A hematoxylin and eosin (H&E) stain of each TNBC tissue specimen was evaluated first to assess tissue histology and preservation quality. One Control Cell Line Slide from PD-L1 IHC 22C3 pharmDx (Code SK006) was included in each IHC staining run conducted and stained with the PD-L1 primary antibody using the PD-L1 IHC staining procedure previously described13. Control slides are included in PD-L1 IHC 22C3 pharmDx with each Control Cell Line Slide containing sections of two pelleted, FFPE cell lines: one cell line with moderate PD-L1 protein expression (NCI-H226) and one cell line with negative PD-L1 protein expression (MCF-7: Michigan Cancer Foundation-7)13,23. Following completion of each staining run, both pellets on the Control Cell Line Slide were examined to confirm proper function of all reagents and met acceptance criteria outlined in the Instructions for Use for PD-L1 IHC 22C3 pharmDx13. Finally, the Negative Control Reagent-stained slide of each TNBC tissue specimen was examined to confirm absence of cell membrane staining, verifying the specific labeling of the target antigen by the primary antibody. All nonspecific staining on the Negative Control Reagent-stained slides was ≤ 1 +.
TNBC tissue specimens were divided into PD-L1 expression levels based on the CPS ≥ 10 clinical diagnostic cutoff: CPS < 10 (Figure 1) and CPS ≥ 10 (Figures 2-4)13. The entire slide of each TNBC specimen stained with the PD-L1 primary antibody was evaluated to verify all viable tumor cells were included in the PD-L1 scoring assessment. A minimum of 100 viable tumor cells were present in the PD-L1 stained slide for the specimen to be considered adequate for PD-L1 evaluation using the CPS algorithm. When defining CPS, certain cell types and tissue structures were either included or excluded for accurate reporting of CPS in TNBC (Table 1).
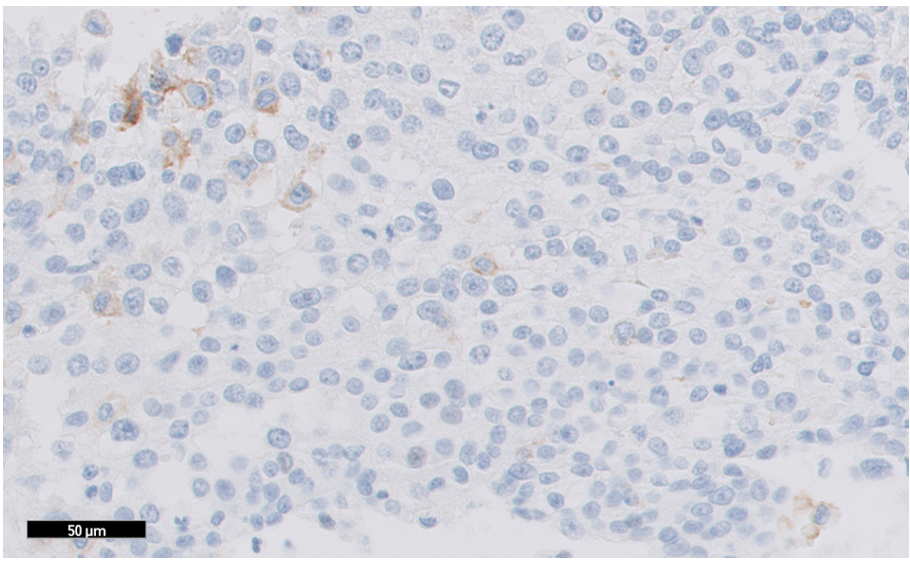
Figure 1: TNBC tissue specimen (at 20x magnification) stained using PD-L1 IHC 22C3 pharmDx and demonstrating CPS < 10 expression. This image represents CPS of 2.
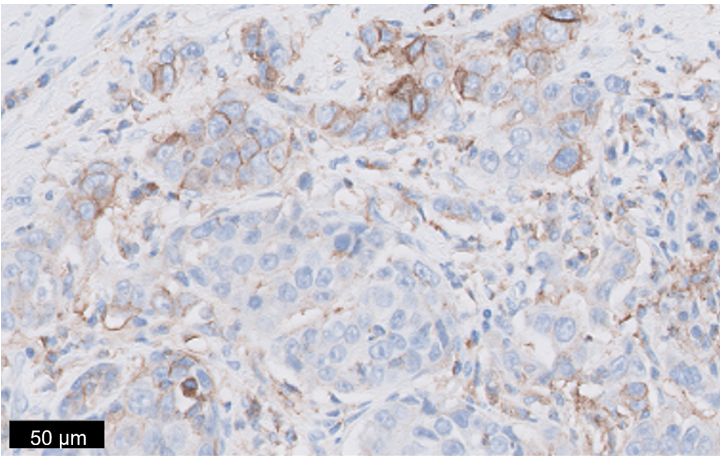
Figure 2: TNBC tissue specimen (at 20x magnification) stained using PD-L1 IHC 22C3 pharmDx and demonstrating CPS ≥ 10 expression. This image represents CPS of 30.
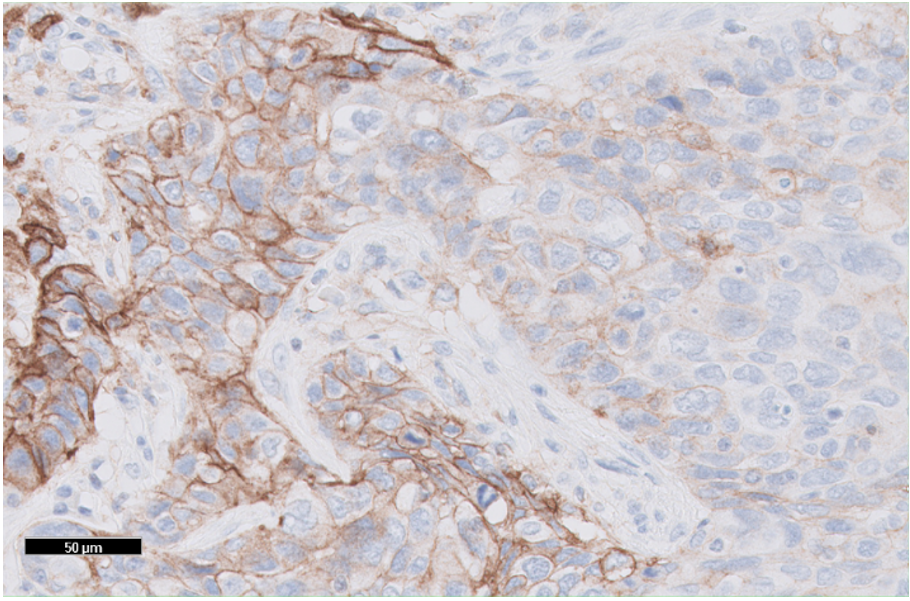
Figure 3: TNBC tissue specimen (at 20x magnification) stained using PD-L1 IHC 22C3 pharmDx and demonstrating CPS ≥ 10 expression. This image represents CPS of 60.
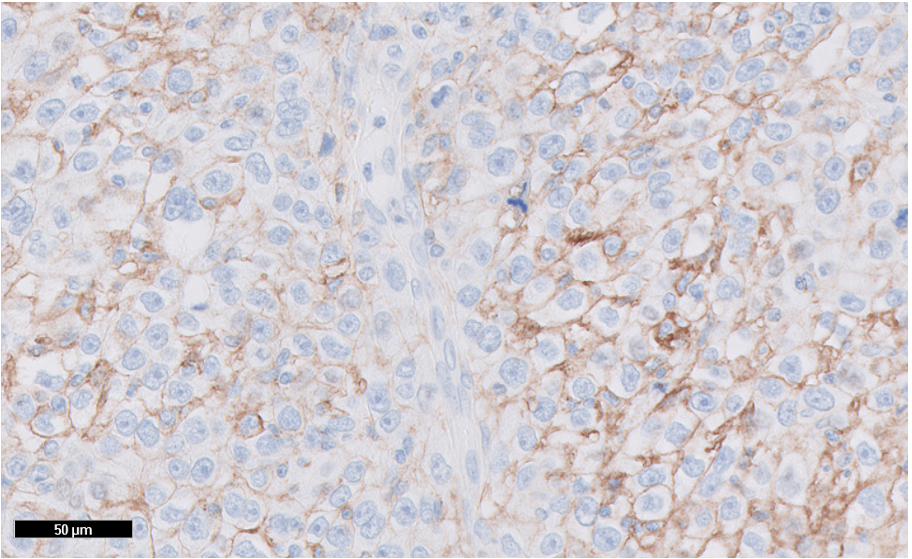
Figure 4: TNBC tissue specimen (at 20x magnification) stained using PD-L1 IHC 22C3 pharmDx and demonstrating CPS ≥ 10 expression. This image represents CPS of 94.
Table 1: CPS numerator and denominator inclusion/exclusion criteria for TNBC.
DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ.
|
Tissue Elements |
Numerator |
Denominator |
|
|
Tumor Cells |
Included |
· Convincing partial or complete linear membrane staining (at any intensity) of viable invasive tumor cells |
All viable invasive tumor cells |
|
Excluded |
· Non-staining tumor cells · Tumor cells with only cytoplasmic staining · Carcinoma in situ (DCIS and LCIS) |
· Non-viable tumor cells · Carcinoma in situ (DCIS and LCIS) |
|
|
Immune Cells |
Included |
· Membrane and/or cytoplasmica staining (at any intensity) of mononuclear inflammatory cells (MICs) within tumor nests and adjacent supporting stromab: o Lymphocytes (lymphocyte aggregates) o Macrophagesc · Only MICs directly associated with the response to the tumor are scored. |
Not included |
|
Excluded |
· Non-staining MICs · MICs associated with DCIS and LCIS · MICs associated with benign structures · MICs (including lymphoid aggregates) not directly associated with the response to the tumor · Neutrophils, eosinophils, and plasma cells |
All immune cells |
|
|
Other Cells |
Included |
Not included |
|
|
Excluded |
· Benign epithelial cells · Stromal cells (including fibroblasts) · Necrotic cells and/or cellular debris |
||
|
aIn MICs, membrane and cytoplasmic staining are often indistinguishable due to high nuclear to cytoplasmic ratio. Therefore, membrane and/or cytoplasmic staining of MICs is included in the score. bAdjacent MICs are defined as being within the same 20x field as the tumor. However, MICs that are NOT directly associated with the response against the tumor should be excluded. cMacrophages and histiocytes are considered the same cells |
|||
CPS was defined using the equation:

Although the result of the CPS calculation can exceed 100, the maximum score was defined as CPS 100
Blinding and randomizing procedure
For validity of results and reduction of scoring recall bias, identifiers for specimens used in all studies were blinded to the observer. Slides were blinded by an individual independent of scoring according to the predetermined blinding and randomizing procedure for each study. For studies where observers scored a set of slides more than once, multiple replicates of the same specimen, or multiple studies with overlapping specimens, a minimum washout period of 14 days was applied. As an additional aid to mitigate the potential for recall bias, inter-/intra-observer reproducibility studies included “wildcard” specimens in each set of slides where observers participated in more than one instance of scoring according to study design. All wildcard specimens were prespecified to be excluded from data analysis.
Statistical analysis
Comparisons between the IHC status [CPS ≥ 10 (positive) or CPS < 10 (negative)] of each test condition and the consensus (most frequently occurring diagnostic observation within a specimen) were made for each specimen. The comparisons to consensus, pooled from all specimens, were then used to calculate percent agreements. Negative percent agreement (NPA), positive percent agreement (PPA), and overall percent agreement (OA) were calculated for each validation study with corresponding two-sided 95% percentile bootstrap confidence intervals (CIs). The percentile bootstrap method cannot compute CIs if 100% agreement is observed. Therefore, for studies resulting in zero discordant comparisons, the Wilson score method was used to compute CIs for the agreement parameter(s) in which 100% agreement was observed.
Staining and scoring of the assay were considered precise and/or reproducible if the lower-bound of the two-sided 95% CIs computed on NPA, PPA, and OA met or exceeded 85%.
Results
PD-L1 IHC 22C3 pharmDx with CPS detects PD-L1 across a range of expression in TNBC specimens
The prevalence of PD-L1 protein in FFPE human TNBC specimens was assessed using PD-L1 IHC 22C3 pharmDx (Figure 5) on 100 archival, whole tissue TNBC specimens, including primary tumors and metastases, early (Stage I-II) and late (Stage III-IV) stage disease. All 100 TNBC specimens were from unique patients of origin. PD-L1 expression was observed from CPS 0 to CPS 100. Using the CPS ≥ 10 cutoff, 70% of specimens had CPS < 10 and 30% of specimens had CPS ≥ 10.
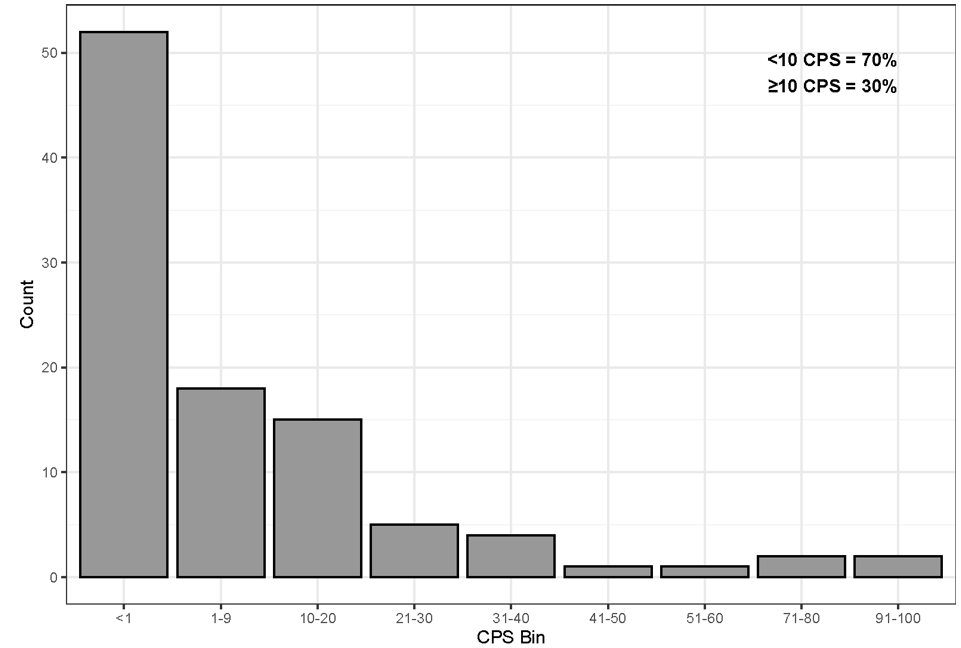
Figure 5: Prevalence of PD-L1 protein in FFPE human TNBC specimens. One hundred TNBC tissues stained using PD-L1 IHC 22C3 pharmDx with 70% of specimens CPS < 10 and 30% of specimens at CPS ≥ 10.
PD-L1 IHC 22C3 pharmDx with CPS detects PD-L1 precisely and repeatably in TNBC specimens
Internal validation studies performed at Agilent Technologies included a sample size of 33 specimens with multiple replicates, depending on the study. Assay precision was measured based on combined precision (inter-operator/instrument/day/lot) and intra-run repeatability using the CPS ≥ 10 cutoff. These studies were analyzed based on percent agreement of diagnostic outcomes (CPS ≥ 10 or CPS < 10) between replicates of each specimen. Combined precision was evaluated by investigating the integrated effect of inter-operator, inter-instrument, inter-day, and inter-lot variables20. All endpoints (NPA/PPA/OA) for combined precision resulted in high agreement at the CPS ≥ 10 cutoff (Table 2 and Figure 6). The following CI lower-bound values were obtained for this study, respectively: 94.3% (NPA), 90.4% (PPA), and 96.3% (OA). Intra-run repeatability was evaluated using six serial sections from each specimen tested with PD-L1 IHC 22C3 pharmDx on one instrument simultaneously; five of the six sections were stained with the primary antibody, with the remaining section stained with Negative Control Reagent. Intra-run repeatability endpoints similarly resulted in high agreement at the CPS ≥ 10 cutoff (Table 2 and Figure 6) with CI lower-bound values of 95.4% (NPA), 86.9% (PPA), and 93.3% (OA).
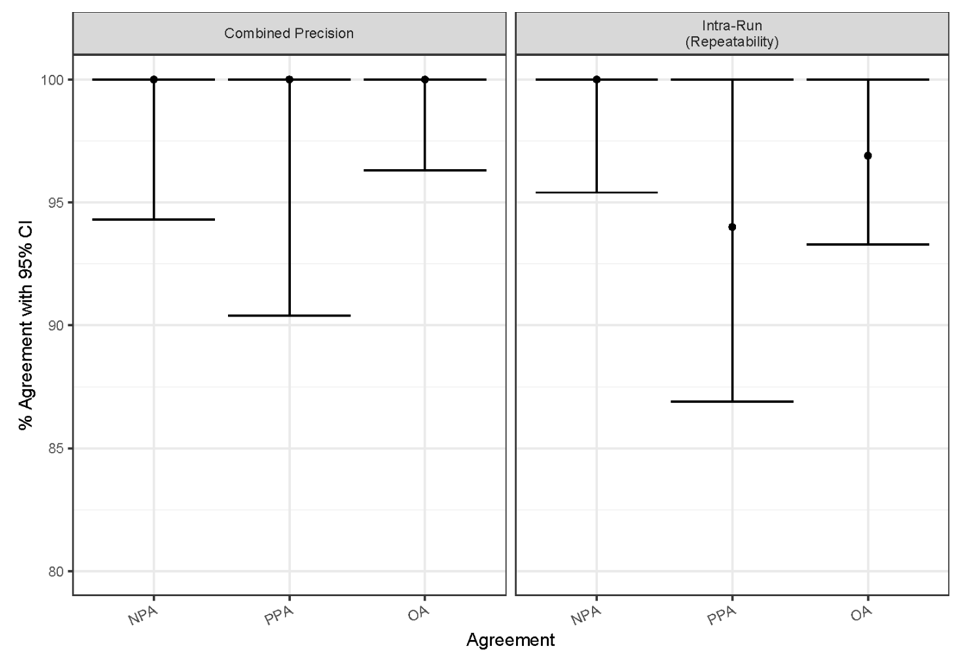
Figure 6: Summary of percent agreement for PD-L1 IHC 22C3 pharmDx internal validation studies. Combined precision and intra-run (repeatability) study parameters met acceptance criteria. CI, confidence interval; NPA, negative percent agreement; PPA, positive percent agreement; OA, overall percent agreement.
Table 2: Point estimates, lower-bound CIs, and upper-bound CIs for TNBC internal and external validation. CI, confidence interval; NPA, negative percent agreement; PPA, positive percent agreement; OA, overall percent agreement.
|
Validation |
Study |
Performance Criteria |
Point Estimate |
95% CI (Bootstrap or Wilsona) |
|
|
Lower-bound CI: 2.5% |
Upper-bound CI: 97.5% |
||||
|
Internal |
Combined precision |
NPA |
100.0% |
94.3% |
100.0% |
|
PPA |
100.0% |
90.4% |
100.0% |
||
|
OA |
100.0% |
96.3% |
100.0% |
||
|
Intra-run repeatability |
NPA |
100.0% |
95.4% |
100.0% |
|
|
PPA |
94.0% |
86.9% |
100.0% |
||
|
OA |
96.9% |
93.3% |
100.0% |
||
|
External |
Inter-Site |
NPA |
93.0% |
85.3% |
100.0% |
|
PPA |
92.1% |
86.3% |
97.1% |
||
|
OA |
92.5% |
87.8% |
96.7% |
||
|
Intra-Site |
NPA |
97.1% |
94.3% |
99.3% |
|
|
PPA |
94.4% |
90.0% |
98.1% |
||
|
OA |
95.7% |
92.7% |
98.2% |
||
|
Inter-Observer |
NPA |
97.0% |
93.6% |
100.0% |
|
|
PPA |
95.4% |
91.2% |
98.7% |
||
|
OA |
96.1% |
93.3% |
98.5% |
||
|
Intra-Observer |
NPA |
98.7% |
96.6% |
100.0% |
|
|
PPA |
96.7% |
94.6% |
98.7% |
||
|
OA |
97.6% |
96.1% |
98.9% |
||
|
aThe percentile bootstrap method cannot compute CIs if 100% agreement is observed. Therefore, for studies resulting in zero discordant comparisons, the Wilson score method was used to compute CIs. |
|||||
PD-L1 IHC 22C3 pharmDx staining and scoring of TNBC specimens is reproducible within a single site and across multiple sites
External reproducibility studies, inter-/intra-site and inter-/intra observer, for CPS ≥ 10 at all endpoints met the predetermined acceptance criteria (Table 2 and Figure 7). Inter-/intra-site external reproducibility was conducted at three separate CAP-accredited/CLIA-licensed laboratories to evaluate the CPS ≥ 10 cutoff (Figure 8a). One operator from each laboratory performed three automated IHC staining runs per round (one iteration of the specimen set) using PD-L1 IHC 22C3 pharmDx, over 5 non-consecutive days. A total of five rounds were conducted with each set of three staining runs associated with one round containing replicate sections from the same set of FFPE TNBC specimens (N=40). Staining was evaluated using the CPS ≥ 10 cutoff by a single pathologist at each of the three external laboratories. Inter-site reproducibility was evaluated by testing the reproducibility between sites, across each of the five testing rounds. For inter-site reproducibility at the CPS ≥ 10 cutoff (Table 2 and Figure 7), the following CI lower-bound values were obtained: 85.3% (NPA), 86.3% (PPA), and 87.8% (OA). Intra-site reproducibility was evaluated by testing the reproducibility within each of the three sites, across each of the five testing rounds. Results for intra-site reproducibility at the CPS ≥ 10 cutoff reflected high agreement (Table 2 and Figure 7) with CI lower-bound values of 94.3% (NPA), 90.0% (PPA), and 92.7% (OA).
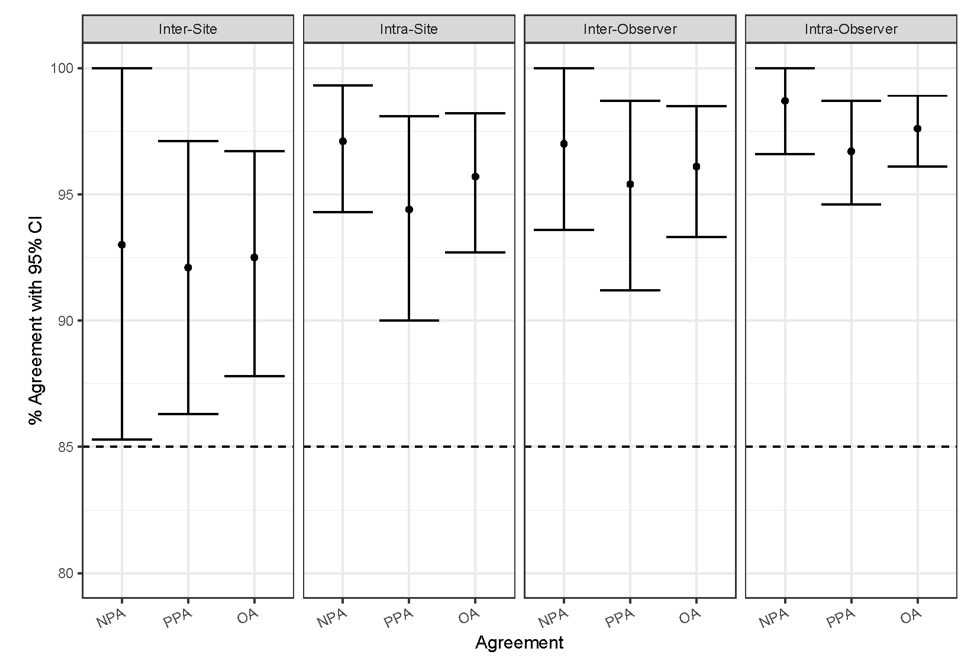
Figure 7: Summary of percent agreement for PD-L1 IHC 22C3 pharmDx external validation studies. Inter-site, intra-site, inter-observer, and intra-observer study parameters met acceptance criteria. CI, confidence interval; NPA, negative percent agreement; PPA, positive percent agreement; OA, overall percent agreement.
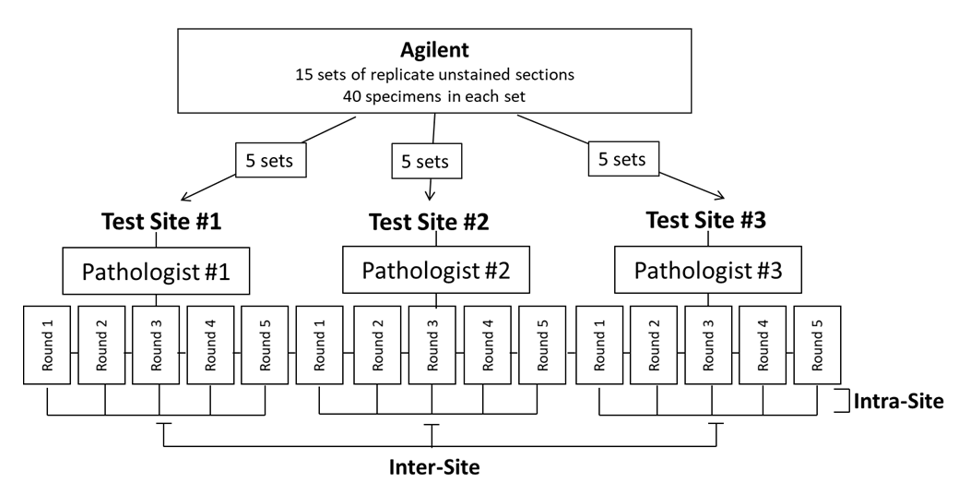
Figure 8a: External Reproducibility Study Design. Study design for inter-/intra-site external reproducibility.
Inter-observer and intra-observer scoring reproducibility were assessed through blinded and randomized slide evaluation at the same three external sites for the CPS ≥ 10 cutoff (Figure 8b). The slide set for evaluation was created using 60 FFPE TNBC specimens stained with PD-L1 IHC 22C3 pharmDx at Agilent Technologies and represented a range of PD-L1 expression using CPS. One trained and certified pathologist (independent from the pathologist used in the inter-site/intra-site study) at each of the three sites performed three independent reads of the TNBC set. The data were analyzed for inter-observer reproducibility (evaluated by testing scoring agreement between the three pathologists, across each of three reads per pathologist) and intra-observer reproducibility (evaluated by testing scoring agreement within each of the three pathologists, across three reads). Results for CPS ≥ 10 inter-observer and intra-observer, respectively, showed high agreement (Table 2 and Figure 7) for all endpoints and CI lower-bound values: 93.6% (NPA), 91.2% (PPA), and 93.3% (OA); 96.6% (NPA), 94.6% (PPA), and 96.1% (OA).
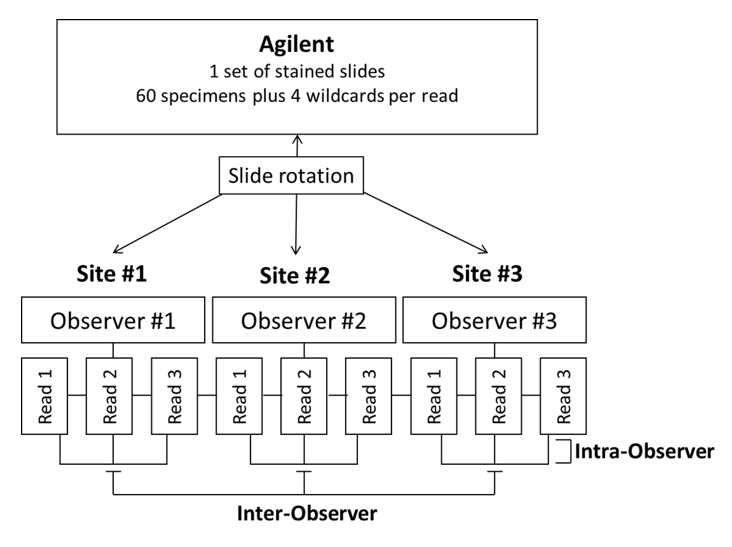
Figure 8b: Study design for inter-/intra-observer external reproducibility.
Discussion
PD-L1 expression in TNBC patients is correlated with decreased overall survival and therefore can be utilized as a target to encourage anti-tumor immune response in the host.5 Pembrolizumab is an immunotherapy treatment for targeting the PD-1:PD-L1 pathway by blocking the interaction between PD-1 on T-cells and PD-L1 on malignant cells. Pembrolizumab, in combination with chemotherapy, is indicated for the treatment of patients with locally recurrent unresectable or metastatic TNBC whose tumors express PD-L1 (CPS ≥ 10) as determined by an FDA approved test18, 19.
The ability of PD-L1 IHC 22C3 pharmDx to detect the target protein, PD-L1, across CPS 0 to CPS 100 ensures that the device can be used for patients whose tumors express PD-L1 at all levels (Figure 5). Previously, the evaluation of Clone 22C3 immunoreactivity in 31 FFPE normal human tissue samples was conducted to support PD-L1 prevalence13. This study supports the ability of PD-L1 IHC 22C3 pharmDx to accurately detect the target substance, PD-L1, localized in cell membranes of normal human tissue samples (e.g., bone marrow megakaryocytes, lung alveolar macrophages, prostate epithelium, tonsil crypt epithelium, etc.)13. These data enhance reliance of PD-L1 IHC 22C3 pharmDx as an ample detection system for the target protein, PD-L1, therefore increasing confidence in the qualification of PD-L1 expression in TNBC tissue assessed using CPS.
In the US, the CPS algorithm is established in the cancer CDx field, with esophageal squamous cell carcinoma (ESCC), cervical cancer, head and neck squamous cell carcinoma (HNSCC), and now TNBC tissues all utilizing this scoring method for determination of PD-L1 protein expression using PD-L1 IHC 22C3 pharmDx 13, 15. All pathologists and observers used for scoring TNBC validation studies (internal and external) were trained according to specified internal training methodologies and tested according to predetermined acceptance criteria. The pathologist training consisted of a detailed overview of the assay performance and a glass slide review specific to the tissue type and cutoff. Following training completion, effectiveness testing is designed to assess intra-observer agreement (concordance of the trainee against themselves) and inter-observer agreement (concordance of the trainee against a consensus). Effectiveness testing is determined using acceptance criteria where OA for both parameters (inter- and intra-observer agreement) must be ≥ 85%. Understanding nuances of TNBC tissue and PD-L1 expression using CPS ≥ 10 contributes to trusted validation results and is essential for assigning an appropriate clinical diagnostic result.
Utilizing multiple sections of the same tissue specimen for combined precision and intra-run repeatability studies requires consideration of the tissue specimen’s tumor heterogeneity. Because IHC relies on detection of protein expression within a small section of the overall extracted tumor, heterogeneity presents potential challenges to the ability for PD-L1 IHC 22C3 pharmDx to detect PD-L1 expression. A study presented by Kalpakoff and colleagues tested the functionality of PD-L1 IHC 22C3 pharmDx across various types of tumor heterogeneity using multiple tumor indications including TNBC24. TNBC intra-block heterogeneity evaluated the performance of PD-L1 IHC 22C3 pharmDx across multiple sections spanning at least 150 µm within each specimen24. For TNBC CPS ≥ 10, 34 specimens representing a dynamic range of PD-L1 expression (CPS 0 to CPS 100) were analyzed with sections taken from the approximate anterior, middle, and posterior of the tissue block24. Results of this study indicated high overall agreement, suggesting that intra-block heterogeneity does not impact the diagnostic status of PD-L1 in TNBC tissues stained with PD-L1 IHC 22C3 pharmDx and that the assay will continue to reliably inform on PD-L1 diagnostic status at the CPS ≥ 10 cutoff for TNBC despite tumor heterogeneity variability24.
For TNBC patient tumors expressing PD-L1 with CPS ≥ 10, pembrolizumab in combination with chemotherapy (paclitaxel, paclitaxel protein-bound, or gemcitabine and carboplatin) demonstrated significant improvement in PFS with a reduced risk of disease progression or death by 35% and a median PFS of 9.7 months compared to 5.6 months utilizing the same chemotherapy methods without pembrolizumab18, 19. PD-L1 IHC 22C3 pharmDx has now been FDA-approved for use as an aid in identifying TNBC patients for treatment with pembrolizumab, based on PD-L1 biomarker expression at the CPS ≥ 10 cutoff18.
PD-L1 IHC 22C3 pharmDx was rigorously tested using TNBC FFPE tissues by conducting internal and external validation studies across various laboratory conditions. These studies were performed intending to mimic clinical laboratory settings and demonstrate the robust reproducibility of PD-L1 IHC 22C3 pharmDx when subjected to typical variables present in standard clinical practice. Average day-to-day testing of PD-L1 IHC 22C3 pharmDx was implemented during combined precision where study results indicated effective testing of three PD-L1 IHC 22C3 pharmDx kit lots, Autostainer Link 48 instruments, and operators all on non-consecutive days. PD-L1 IHC 22C3 pharmDx inter-observer and intra-observer study results emphasized the scoring reproducibility of PD-L1 in TNBC tissue specimens at a CPS ≥ 10 cutoff when scored by different observers as well as by a single observer. Additionally, PD-L1 IHC 22C3 pharmDx reproducibility over multiple days and runs within one clinical laboratory and across multiple (3) clinical laboratories was effectively demonstrated with inter- and intra-site study parameters executed during external validation. The success of these studies further provides clinicians confidence when diagnosing disease and prescribing personalized treatment to their patients.
Conclusions
Validation studies and corresponding data presented supporting PD-L1 IHC 22C3 pharmDx in TNBC FFPE tissues was collected utilizing a scoring algorithm that does not require separate calculations of the tumor and immune cell components. Therefore, analyses comparing the contribution of PD-L1 staining tumor cells versus PD-L1 staining immune cells to the CPS were not conducted. Future studies exploring PD-L1 expression in tumor cells versus immune cells could provide valuable insight into CPS trends and personalized treatment. Furthermore, the studies presented were conducted using only TNBC FFPE tissues to support the FDA-approval of PD-L1 IHC 22C3 pharmDx in TNBC tissue as a companion diagnostic and therefore other subtypes of breast cancer were not included in these analyses. Research utilizing PD-L1 IHC 22C3 pharmDx in combination with the CPS algorithm and cutoff(s) to evaluate PD-L1 expression in non-TNBC breast cancers may significantly advance personalized treatment options for breast cancer patients.
All studies presented herein independently exceeded pre-specified criteria of the lower-bound of the two-sided 95% CIs at or above 85%, demonstrating that PD-L1 IHC 22C3 pharmDx is a precise and reproducible assay when using the CPS ≥ 10 cutoff on FFPE TNBC tissue specimens. The parameters across internal and external validation studies have been used to analytically validate the PD-L1 IHC 22C3 pharmDx device supporting its intended use in TNBC FFPE tissues. In conclusion, the data presented supports PD-L1 IHC 22C3 pharmDx device precision and reproducibility in TNBC FFPE tissues, increasing confidence for clinical use of the device.
List of Abbreviations
Triple-negative breast cancer (TNBC), estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor type 2 (HER2), programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), companion diagnostic (CDx), immunohistochemistry (IHC), formalin-fixed, paraffin-embedded (FFPE), non-small cell lung cancer (NSCLC), esophageal squamous cell carcinoma (ESCC), head and neck squamous cell carcinoma (HNSCC), Combined Positive Score (CPS), gastroesophageal junction (GEJ), progression-free survival (PFS), Food and Drug Administration (FDA), College of American Pathologists (CAP), Clinical Laboratory Improvements Amendments (CLIA), Institutional Review Board (IRB), hematoxylin and eosin (H&E), Negative percent agreement (NPA), positive percent agreement (PPA), overall percent agreement (OA), confidence interval (CI).
Conflicts of Interest
All authors are current or former employees of Agilent Technologies, Inc.
The authors acknowledge Merck & Co., Inc. (Rahway, NJ, USA) for their long-term partnership and funding for these studies. We acknowledge the contributions of Lars Rudbeck as Scientific Editor for Agilent Technologies. We acknowledge the following investigators for their participation and expertise in the examination of tissue specimens included in internal validation studies: Edward Manna, Holly Yamada, and Micki Adams. We acknowledge the following institutions for their participation as test sites in external validation studies: Hematogenix (8150 W 185th St, Tinley Park, IL 60487, USA), ARUP (500 Chipeta Way, Salt Lake City, UT 84108, USA), and Mosaic Laboratories (12 Spectrum Pointe Dr, Lake Forest, CA 92630, USA). We acknowledge Dr. AF Gazdar and Dr. JD Minna at NIH for their contribution in developing NCI-H226 (ATCC Number: CRL-5826). We acknowledge the following contributions for tissue samples used in these studies and tissue images provided within this manuscript: Samples/tissue supplied by Conversant; Cooperative Human Tissue Network which is funded by the National Cancer Institute (Other investigators may have received specimens from the same subjects); Tissue samples supplied by BioIVT (Hicksville, NY, USA); The data and biospecimens used in this project was provided by Centre Hospitalier Universitaire (CHU) de Nice with appropriate ethics approval and through Azenta Life Sciences; The data and biospecimens used in this project was provided by Contract Research Ltd with appropriate ethics approval and through Azenta Life Sciences; The data and biospecimens used in this project was provided by DRCRO Pakistan with appropriate ethics approval and through Azenta Life Sciences; The data and biospecimens used in this project was provided by Hospices Civils de Lyon with appropriate ethics approval and through Azenta Life Sciences; Biological materials were provided by the Ontario Tumour Bank, which is supported by the Ontario Institute for Cancer Research through funding provided by the Government of Ontario.; The data and biospecimens used in this project was provided by Centre Antoine Lacassagne (CAL) with appropriate ethics approval and through Azenta Life Sciences.
References
- Jia H, Trucia C, Wang B, et al. Immunotherapy for triple-negative breast cancer: Existing challenges and exciting prospects. Drug Resist Updat. 2017; 32:1-15.
- Li X, Yang J, Peng L, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat. 2017;161(2):279-287.
- Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(6):619-626.
- NedeljkoviÄ M, DamjanoviÄ A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells. 2019;8(9):957.
- Muenst S, Soysal SD, Gao F, et al. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139(3):667-676.
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027-1034.
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2(2):116-126.
- Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8(3):239-245. doi:10.1038/ni1443
- Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14 (4):847-856.
- Dermani FK, Samadi P, Rahmani G, et al. PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy. J Cell Physiol. 2019;234(2):1313-1325.
- Guo H, Ding Q, Gong Y, et al. Comparison of three scoring methods using the FDA-approved 22C3 immunohistochemistry assay to evaluate PD-L1 expression in breast cancer and their association with clinicopathologic factors. Breast Cancer Res. 2020;22(1):69.
- Roach C, Zhang N, Corigliano E, et al. Development of a Companion Diagnostic PD-L1 Immunohistochemistry Assay for Pembrolizumab Therapy in Non-Small-cell Lung Cancer. Appl Immunohistochem Mol Morphol. 2016;24(6):392-397.
- PD-L1 IHC 22C3 pharmDx [Instructions for Use]. November 29, 2022. Accessed December 2, 2022. Available from: www.agilent.com/library/eifu. Code SK006.
- Kulangara K, Zhang N, Corigliano E, et al. Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer. Arch Pathol Lab Med. 2019;143(3):330-337.
- La Placa CJ, Vilardo MD, Watts BM, et al. Development of a Companion Diagnostic PD-L1 Immunohistochemistry Assay for Pembrolizumab Therapy in Head and Neck Squamous Cell Carcinoma. J Cancer Treatment Diagn. 2021;5(1):9-17
- Merck’s KEYTRUDA® (pembrolizumab) Plus Chemotherapy Demonstrated Statistically Significant Improvement in Progression-Free Survival Versus Chemotherapy in Certain Patients with Metastatic Triple-Negative Breast Cancer [Merck & Co., Inc. News website]. May 13, 2020. Accessed July 21, 2023. Available from: https://www.merck.com/news/mercks-keytruda-pembrolizumab-plus-chemotherapy-demonstrated-statistically-significant-improvement-in-progression-free-survival-versus-chemotherapy-in-certain-patients-with-metastatic/
- Cortes J, Cescon DW, Rugo HS, et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol. 2020; 38(15_suppl):1000-1000
- FDA Approves Merck’s KEYTRUDA® (pembrolizumab) in Combination with Chemotherapy for Patients with Locally Recurrent Unresectable or Metastatic TripleâNegative Breast Cancer Whose Tumors Express PD-L1 (CPS ≥10) [Merck & Co., Inc. News web site]. November 13, 2020. Accessed November 14, 2022. Available from: https://www.merck.com/news/fda-approves-mercks-keytruda-pembrolizumab-in-combination-with-chemotherapy-for-patients-with-locally-recurrent-unresectable-or-metastatic-triple%e2%80%91negative-breast-cancer-whose/.
- Keytruda [package insert]. Rahway, NJ: Merck & Co., Inc.; 2022.
- Hewitt S, Robinowitz M, Bogen S, et al. Quality Assurance for Design Control and Implementation of Immunohistochemistry Assays, CLSI Document, 2nd Edition. I/LA28-A2 2011, 31(4).
- Dako ER/PR pharmDx kit [Instructions for Use]. October 11, 2021. Accessed July 21, 2023. Available from: www.agilent.com/library/eifu. Code SK310.
- HercepTest for Automated Link Platforms [Instructions for Use]. November 21, 2021. Accessed July 21, 2023. Available from: www.agilent.com/library/eifu. Code SK001.
- Soule HD, Vazquez J, Long A, et al. A Human Cell Line from a Pleural Effusion Derived from a Breast Carcinoma. J Natl Cancer Inst. 1973;51(5):1409-1416.
- Kalpakoff M, Hund S, Musser J, et al. Intrapatient Tumor Heterogeneity in IHC Interpretation Using PD-L1 IHC 22C3 pharmDx. Appl Immunohistochem Mol Morphol. 2021;29(9):667-673.
