B7 Family Proteins in Cancer Progression: Immunological and Non-Immunological Functions
Qin Ye1, Jiayang Liu2, Ke Xie1*
1Department of Oncology, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan 610054, P.R. China
2State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, and Collaborative Innovation Center for Biotherapy, Chengdu, 610041, P.R. China
Abstract
The B7 family of proteins is commonly divided into three classes according to their structure and the type of receptor they bind to. The B7 proteins exhibit both positive and negative functions with regard to the immune response and are known to be co-inhibitory or co-stimulatory ligands that regulate antitumor immune responses. They are also involved in the regulation of cancer progression via non-immunological functions such as accelerating metabolism, promoting proliferation, and facilitating chemoresistance. Given the dynamic interaction between cancer cells and B7 family proteins, each member has been considered as a novel biomarker or therapeutic target that may well improve the effectiveness of cancer diagnosis and treatment. In this review, we summarize the characteristics of B7 proteins and their immunological and non-immunological roles in cancer progression.
Introduction
Despite advances in the diagnosis and treatment of different cancers over the past decades, the overall prognosis for patients with most cancer types remains poor due to metastasis. In most cases, metastatic tumor cells develop strategies to evade immunosurveillance and resist therapeutic drugs1. These strategies include expressing aberrant, tumor-specific antigens, either by inducing survival signals from tumor-infiltrating immune cells or driving immunosuppression2. To be effective, T cell-mediated antitumor immunity needs to make a positive identification of cancer-associated antigens. The process of identification requires the interaction between specific receptors on T cells and the major histocompatibility complex (MHC), and additional co-stimulatory or co-inhibitory signals arising from antigen-presenting cells (APCs) such as macrophages and dendritic cells, other immunocytes, stromal, and tumor cells3,4. Education and modulation of tumor-infiltrating immune cells and cancer cells are considered as a promising antitumor strategy.
In the wake of the effective treatment of cancer with immune checkpoint inhibitors, the B7 family of molecules has received increasing attention in recent years5,6. Ten B7 family members have been identified so far, including B7-1 (also known as CD80), B7-2 (also known as CD86), B7-H2 (also known as CD275 or ICOSL), B7-H1 (also known as CD274 or PD-L1), B7-DC (also known as CD273 or PD-L2), B7-H3 (also known as CD276), B7-H4 (also known as B7S1, B7x, or Vtcn1), B7-H5 (also known as VISTA, GI24, Dies1 or PD-1H), B7-H6 (also known as NCR3LG1) and B7-H7 (also known as HHLA2)7,8. The B7 family is composed of structurally related, cell-surface, proteins that regulate immune responses by delivering co-inhibitory or co-stimulatory signals through their receptors9. Manipulation of the signals delivered by B7 ligands has now shown great potential in the treatment of cancer. Additionally, B7 family members are also emerging as important regulators involved in tumor growth, invasion, metastasis, and drug sensitivity independent of the immune system6. In this review, we summarize the characteristics of B7 family proteins and their immunological and non-immunological functions in cancer progression with a view to promoting promising anticancer strategies.
The Characteristics of B7 Proteins and their Roles in Tumor Immunology
Class I B7 molecules: B7-1, B7-2 and B7-H2
Class I B7 molecules are ligands of the CD28 family of receptors, which are expressed on activated APCs, T lymphocytes and tumor cells, and mediate dynamic interactions between cancer cells and the host immune system.
B7-1 and B7-2 (expressed on APCs) function as immune regulators by binding to CD28 on T cells or to the inhibitory receptor, CTLA-4,10. CD28 co-stimulatory or CTLA-4 co-inhibitory signals are required for the activation or inhibition of naïve T cells, respectively11. The process of T cell activation is initiated by T cell antigen receptor (TCR) engaged by the cognate antigenic peptide-MHC complex on APCs together with CD28 co-stimulation by B7-1 and B7-212. The cascade of stimulation signaling is able to convert naïve T cells into effector cells via clonal expansion and differentiation, resulting in cytokine production, blockade of apoptosis and entry into the cell cycle. In contrast, T cells become anergic and non-responsive upon binding of the ligands to the inhibitory receptor, CTLA-413.
B7-H2 is another co-stimulatory member of the B7 family. B7-H2, which is expressed in non-lymphoid tissues, B cells, macrophages, and solid tumor cells, binds to another member of the CD28 family, the inducible T-cell co-stimulator (ICOS, CD278) to regulate the immune response14. This binding drives naïve T cells to differentiate into cytotoxic T lymphocytes that can attack cancer cells resulting in tumor regression11. It has been shown that disrupting the regulatory role of miR-24 on B7-H2 expression via the SNP rs4819388 in the B7-H2 3'-UTR might contribute to the occurrence of gastric cancer15. However, Tamura et al. found that B7-2 and B7-H2 molecules were constitutively expressed on acute myeloid leukemia (AML) cells and promoted AML pathogenesis by facilitating the escape of tumor cells from immune surveillance16. These previous findings suggest that B7-H2 might mediate different immune responses in different tumors.
Class II B7 molecules: B7-H1 (PD-L1) and B7-DC (PD-L2)
The class II B7 members have a common receptor, programmed cell death-1 (PD-1), which is a member of the immunoglobulin (Ig) superfamily. B7-H1 and B7-DC negatively regulate T cell antigen receptor (TCR) and B-cell antigen receptor (BCR) signaling to maintain peripheral immune tolerance by interacting with PD-117,18. Emerging evidence19 has suggested an important role of class II B7 members in antitumor immune responses. A recent report has shown that the clinical responses of advanced non-small-cell lung cancer (NSCLC) patients to PD-L1/PD-1 blockade monotherapy are dependent on baseline systemic CD4 immunity20. Quandt et al. found that the secretion of IL-4 and TNFα by immune cells in the tumor microenvironment induced the expression of B7-H1 on the surface of renal cell carcinoma cells (RCC), thus suppressing the T cell-mediated antitumor immune response21. Increasing evidence has highlighted a key role of B7-DC (PD-L2) in the suppression of antitumor immune responses by colorectal cancer cells22. However, there also exists compelling evidence for the co-stimulatory function of B7-DC (PD-L2) in a manner independent of PD-1. For example, Liu et al. demonstrated that expression of B7-DC on tumor cells enhanced CD8 T cell-mediated lysis of tumor cells through induction of the effector phase of antitumor immunity. The mechanism of this CD8 T cell killing of tumor cells was PD-1-independent23. In summary, these data suggest that the class II B7 members exhibit potential negative functions for immune regulation via interaction with PD-1or positive effects in a PD-1-independent manner.
Table1. Classification and characteristics of B7 family proteins.
|
B7 Classification |
B7 Member |
Common Name |
Major Receptor |
Function |
Expressed By |
References |
|
I |
B7-1 |
CD80 |
CD28, CTLA-4 |
Positive or negative |
Dendritic cells, macrophages, hematopoietic cells, B cells |
[12] [13] [10] [11] |
|
B7-2 |
CD86 |
|||||
|
B7-H2 |
CD275, ICOS- L |
ICOS |
Positive |
Non-lymphoid tissues, B cells, macrophages, tumor cells |
[14] [15] [16] |
|
|
II |
B7-H1 |
CD274, PD-L1 |
PD-1 |
Negative |
Tumor cells |
[19] [20] |
|
B7-DC |
CD273, PD-L2 |
[21] [22] |
||||
|
III |
B7-H3 |
CD276 |
Not identified |
Negative |
Immune cells, tumor cells |
[28] [27] [26] |
|
B7-H4 |
B7S1, B7x, Vtcn1 |
Tumor cells |
[32] [33] [34] |
|||
|
B7-H7 |
HHLA2 |
CD28H |
Positive |
Tumor cells |
[23] |
|
|
|
B7-H5 |
VISTA, GI24, Dies1, PD-1H |
Not identified |
Negative |
Tumor cells
|
[37] [38] |
|
|
B7-H6 |
NCR3LG1 |
NKp30 |
Positive |
Tumor cells |
[40] [41] |
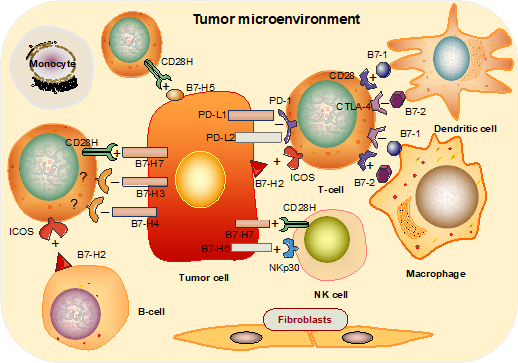
Figure 1: The immune functions of the B7 family of proteins. B7-1 and B7-2 molecules expressed on APCs exhibit positive or negative functions via interaction with either cognate stimulatory receptor, CD28, or inhibitory receptor, CTLA-4, respectively; B7-H2 expressed on B cells and solid tumor cells is mainly activated in T cells via ICOS; B7-H1 and B7-DC negatively regulate the TCR interaction via PD-1; B7-H3, B7-H4 are thought to be T cell inhibitors, while B7H7 acts as a co-stimulator in T cell activation and as a strong activator of NK cells; B7-H5 shows enhanced anti-tumor T cell immunity; B7-H6 binds to NKp30 expressed on NK cells, thus activating anti-tumor cytotoxicity.
Class III B7: B7-H3, B7-H4 and B7-H7
B7-H3 and B7-H4 are type I-transmembrane proteins and their receptors have not been unequivocally identified. A recent report suggested that the CD28 homolog (CD28H), which is expressed on primary natural killer (NK) cells, was a receptor of B7-H719. However, the contributions of B7-H3, B7-H4, B7-H7 to the tumor immune response have not been defined. Whether the function of B7-H3 is co-stimulatory or co-inhibitory remains controversial because of the lack of an identified receptor. Chapoval et al. initially characterized B7-H3 as a T cell-stimulating protein24, but the majority of current research suggests that B7-H3 is a T cell inhibitor25. It is expressed in many tissues and cell types, such as the human gestational trophoblastic neoplasia, GTN26, colorectal cancers (CRC)27 and on the surface of immune cells, specifically APCs28. B7-H3 may be an important immunological target in cancer treatment but until the mechanism of its action is better defined this remains unknown.
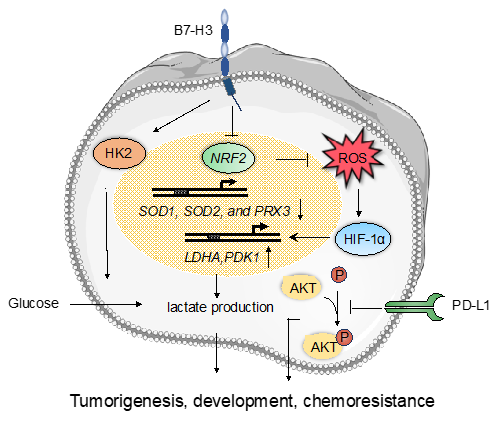
Figure 2: The non-immune functions of the B7 family proteins. B7-H3 is a novel regulator of chemoresistance and glucose metabolism in CRC that increases hexokinase 2 (HK2) expression; B7-H3 promotes glucose uptake and lactate production via increasing the protein levels of HIF1α and the downstream targets LDHA and PDK1; B7-H3 promotes reactive oxygen species-dependent stabilization of HIF1α by suppressing the activity of Nrf2 and its target genes SOD1, SOD2 and PRX3; PD-L1 enhances immune-independent tumor cell proliferation via inhibiting phosphorylation of Akt.
B7-H4 was first identified in 2003 by bioinformatics analysis29, but its receptor has yet to be found. BTLA was initially considered to be the binding partner of B7-H430, but this was not verified in subsequent experiments31. Overexpression of B7-H4 protein has been reported in several human tumors, including breast cancer32, ovarian carcinoma33, and bladder cancer34. B7-H4 was considered as a co-inhibitor of T cell activation and proliferation and Suh et al. revealed that in mice, B7-H4 knockout supported the polarization of T cells35.
B7-H7, also called the HERV-HLTR-associating protein 2 (HHLA2), has been reported to inhibit proliferation and cytokine production of both human CD4 and CD8 T cells36, but a recent report has shown that B7-H7 can act as a co-stimulator of T cell activation and as a strong activator of NK cells, thus producing pro-inflammatory cytokines and inducing lysis of target cancer cells19. Thus, B7-H7 may be either co-stimulatory or co-inhibitory under different conditions.
Novel development of B7: B7-H5 and B7-H6
In addition to the co-stimulatory and co-inhibitory activities discussed above, new roles of B7-H5 and B7-H6 ligands have recently been discovered showing potential for cancer immunotherapy. For example, B7-H5, also known as V-domain Ig suppressor of T cell activation (VISTA), is a B7 checkpoint ligand primarily expressed on hematopoietic cells and dendritic cells. B7-H5 has been showed to selectively co-stimulate human T-cell growth via CD28H, enhance antitumor T cell immunity, and decrease melanoma tumor burden; thus, targeting B7-H5 may provide promising approaches for cancer immunotherapy37-39. B7-H6, also known as natural cytotoxicity-triggering receptor 3 (NCR3LG1), has also been identified as a B7 family ligand40. B7-H6 is expressed on the surface of many types of tumor cells, such as oral squamous cell carcinoma, breast cancer, and glioma41-43. B7-H6 has been shown to bind to the natural killer cell-activating receptor NKp3044 and can activate antitumor cytotoxicity, cytokine production and trigger cytolysis of tumor cells45. Thus, B7-H6 may be potentially useful as a cancer immunotherapy adjunct and a novel cancer biomarker.
Non-Immunological Function of B7 Family Members in Cancer Progression
Although B7 family proteins are well known as immunoregulatory molecules in different types of cancer, another aspect of these proteins that has received less scrutiny is their non-immunological activity. It has been reported that B7-H3 may be a novel regulator of chemoresistance and glucose metabolism in CRC cells. Overexpression of B7-H3 in CRC cells could induce chemoresistance by increasing expression of hexokinase 2 (HK2), a key tumor progression mediator that enhances the rate of glucose consumption and lactate production27. Another study has shown a critical role of B7-H3 in the regulation of breast cancer metabolism via a mechanism separate from the immune system. Overexpressing B7-H3 in breast cancer cells promoted glucose uptake and lactate production via increasing the protein levels of HIF-1α, LDHA, and PDK1, all of which are enzymes in the glycolytic pathway. Furthermore, B7-H3 could promote reactive oxygen species-dependent stabilization of HIF-1α by suppressing the activity of the stress-activated transcription factor, Nrf2, and the expression of its target genes, including the antioxidants SOD1, SOD2, and PRX3, thus contributing to cancer progression46. PD-L1, and other members of the B7 family, have been shown to possess additional tumor-intrinsic effects independent of immunological functions as well. Clark et al. demonstrated that PD-L1 enhanced immune-independent tumor cell proliferation in melanoma and ovarian cancer cells by increasing basal expression and activation of mTORC1 and inhibiting Akt phosphorylation43. Thus, B7 family members are also involved in the regulation of tumor progression independently of the immune system.
Conclusions
The family of B7 proteins is expressed on the surface of various cell types including APCs, immune cells, matrix cells, and tumor cells, while the B7 family receptors are mainly distributed on T cells, B cells, and NK cells. Among the B7 proteins, both co-inhibitory and co-stimulatory ligands have been found to participate in various negative and positive functions related to antitumor immune responses. Given their critical functions in immune modulation, several B7 family members have been tested as drug targets for immunotherapy. For instance, checkpoint-targeted drugs such as anti-PD-1 monoclonal antibody (nivolumab, pembrolizumab), anti-PD-L1 monoclonal antibody (atezolizumab, durvalumab, avelumab) and anti-CTLA-4 monoclonal antibody (ipilimumab) have been approved by the U.S. Food and Drug Administration. Meaningful therapeutic responses have been achieved against melanoma, non-small cell lung cancer, bladder cancer, and leukemia through immune checkpoint blockade therapy. In addition to their vital role in immune regulation, B7 proteins also regulate glucose metabolism, tumor growth, and chemoresistance independent of the immune system. There is an urgent need for clinical trials to assess the potential anticancer efficacy of B7 proteins in order to improve cancer treatment, reduce devastating side effects and identify new biomarkers for cancer immunotherapy.
References
- Cassetta, L. and J.W. Pollard, Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov, 2018. 17(12): p. 887-904.
- Lopez-Soto, A., et al., Control of Metastasis by NK Cells. Cancer Cell, 2017. 32(2): p. 135-154.
- Guerriero, J.L., Macrophages: Their Untold Story in T Cell Activation and Function. Int Rev Cell Mol Biol, 2019. 342: p. 73-93.
- Onda, M., K. Kobayashi, and I. Pastan, Depletion of regulatory T cells in tumors with an anti-CD25 immunotoxin induces CD8 T cell-mediated systemic antitumor immunity. Proc Natl Acad Sci U S A, 2019.
- Harrington, K., et al., Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov, 2019.
- Flem-Karlsen, K., et al., B7-H3 in Cancer - Beyond Immune Regulation. Trends Cancer, 2018. 4(6): p. 401-404.
- Janakiram, M., et al., The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol Rev, 2017. 276(1): p. 26-39.
- Greaves, P. and J.G. Gribben, The role of B7 family molecules in hematologic malignancy. Blood, 2013. 121(5): p. 734-44.
- Croft, M., The evolving crosstalk between co-stimulatory and co-inhibitory receptors: HVEM-BTLA. Trends Immunol, 2005. 26(6): p. 292-4.
- Sharpe, A.H. and G.J. Freeman, The B7-CD28 superfamily. Nat Rev Immunol, 2002. 2(2): p. 116-26.
- Yao, S., et al., B7-h2 is a costimulatory ligand for CD28 in human. Immunity, 2011. 34(5): p. 729-40.
- Feng, Y., E.L. Reinherz, and M.J. Lang, alphabeta T Cell Receptor Mechanosensing Forces out Serial Engagement. Trends Immunol, 2018. 39(8): p. 596-609.
- Chen, L. and D.B. Flies, Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol, 2013. 13(4): p. 227-42.
- Wang, S., et al., Costimulation of T cells by B7-H2, a B7-like molecule that binds ICOS. Blood, 2000. 96(8): p. 2808-13.
- Yang, P., et al., A functional variant at miR-24 binding site in B7-H2 alters susceptibility to gastric cancer in a Chinese Han population. Mol Immunol, 2013. 56(1-2): p. 98-103.
- Tamura, H., et al., Expression of functional B7-H2 and B7.2 costimulatory molecules and their prognostic implications in de novo acute myeloid leukemia. Clin Cancer Res, 2005. 11(16): p. 5708-17.
- Lazar-Molnar, E., et al., The PD-1/PD-L costimulatory pathway critically affects host resistance to the pathogenic fungus Histoplasma capsulatum. Proc Natl Acad Sci U S A, 2008. 105(7): p. 2658-63.
- Xiao, X., et al., PD-1hi Identifies a Novel Regulatory B-cell Population in Human Hepatoma That Promotes Disease Progression. Cancer Discov, 2016. 6(5): p. 546-59.
- Zhuang, X. and E.O. Long, CD28 Homolog Is a Strong Activator of Natural Killer Cells for Lysis of B7H7(+) Tumor Cells. Cancer Immunol Res, 2019. 7(6): p. 939-951.
- Zuazo, M., et al., Functional systemic CD4 immunity is required for clinical responses to PD-L1/PD-1 blockade therapy. EMBO Mol Med, 2019. 11(7): p. e10293.
- Quandt, D., et al., Synergistic effects of IL-4 and TNFalpha on the induction of B7-H1 in renal cell carcinoma cells inhibiting allogeneic T cell proliferation. J Transl Med, 2014. 12: p. 151.
- Masugi, Y., et al., Tumor PDCD1LG2 (PD-L2) Expression and the Lymphocytic Reaction to Colorectal Cancer. Cancer Immunol Res, 2017. 5(11): p. 1046-1055.
- Liu, X., et al., B7DC/PDL2 promotes tumor immunity by a PD-1-independent mechanism. J Exp Med, 2003. 197(12): p. 1721-30.
- Chapoval, A.I., et al., B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol, 2001. 2(3): p. 269-74.
- Pardoll, D.M., The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer, 2012. 12(4): p. 252-64.
- Zong, L., et al., PD-L1, B7-H3 and VISTA are highly expressed in gestational trophoblastic neoplasia. Histopathology, 2019.
- Shi, T., et al., B7-H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death Dis, 2019. 10(4): p. 308.
- Picarda, E., K.C. Ohaegbulam, and X. Zang, Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin Cancer Res, 2016. 22(14): p. 3425-3431.
- Sica, G.L., et al., B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity, 2003. 18(6): p. 849-61.
- Watanabe, N., et al., BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol, 2003. 4(7): p. 670-9.
- Sedy, J.R., et al., B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol, 2005. 6(1): p. 90-8.
- Tringler, B., et al., B7-h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res, 2005. 11(5): p. 1842-8.
- Dangaj, D., et al., Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses. Cancer Res, 2013. 73(15): p. 4820-9.
- Fan, M., et al., B7-H4 expression is correlated with tumor progression and clinical outcome in urothelial cell carcinoma. Int J Clin Exp Pathol, 2014. 7(10): p. 6768-75.
- Suh, W.K., et al., Generation and characterization of B7-H4/B7S1/B7x-deficient mice. Mol Cell Biol, 2006. 26(17): p. 6403-11.
- Zhao, R., et al., HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci U S A, 2013. 110(24): p. 9879-84.
- Le Mercier, I., et al., VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res, 2014. 74(7): p. 1933-44.
- Wang, L., et al., VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med, 2011. 208(3): p. 577-92.
- Zhu, Y., et al., B7-H5 costimulates human T cells via CD28H. Nat Commun, 2013. 4: p. 2043.
- Brandt, C.S., et al., The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med, 2009. 206(7): p. 1495-503.
- Che, F., et al., B7-H6 expression is induced by lipopolysaccharide and facilitates cancer invasion and metastasis in human gliomas. Int Immunopharmacol, 2018. 59: p. 318-327.
- Wang, J., et al., The prognostic value of B7-H6 protein expression in human oral squamous cell carcinoma. J Oral Pathol Med, 2017. 46(9): p. 766-772.
- Clark, C.A., et al., Tumor-Intrinsic PD-L1 Signals Regulate Cell Growth, Pathogenesis, and Autophagy in Ovarian Cancer and Melanoma. Cancer Res, 2016. 76(23): p. 6964-6974.
- Schlecker, E., et al., Metalloprotease-mediated tumor cell shedding of B7-H6, the ligand of the natural killer cell-activating receptor NKp30. Cancer Res, 2014. 74(13): p. 3429-40.
- Fiegler, N., et al., Downregulation of the activating NKp30 ligand B7-H6 by HDAC inhibitors impairs tumor cell recognition by NK cells. Blood, 2013. 122(5): p. 684-93.
- Lim, S., et al., Immunoregulatory Protein B7-H3 Reprograms Glucose Metabolism in Cancer Cells by ROS-Mediated Stabilization of HIF1alpha. Cancer Res, 2016. 76(8): p. 2231-42.
